Dopamine receptor D3 regulates endocytic sorting by a Prazosin-sensitive interaction with the coatomer COPI
- PMID: 22802617
- PMCID: PMC3411939
- DOI: 10.1073/pnas.1207821109
Dopamine receptor D3 regulates endocytic sorting by a Prazosin-sensitive interaction with the coatomer COPI
Abstract
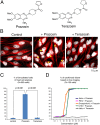
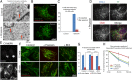
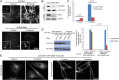
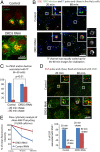
Macromolecules enter cells by endocytosis and are sorted to different cellular destinations in early/sorting endosomes. The mechanism and regulation of sorting are poorly understood, although transitions between vesicular and tubular endosomes are important. We found that the antihypertensive drug Prazosin inhibits endocytic sorting by an off-target perturbation of the G protein-coupled receptor dopamine receptor D(3) (DRD3). Prazosin is also a potent cytokinesis inhibitor, likely as a consequence of its effects on endosomes. Prazosin stabilizes a normally transient interaction between DRD3 and the coatomer COPI, a complex involved in membrane transport, and shifts endosomal morphology entirely to tubules, disrupting cargo sorting. RNAi depletion of DRD3 alone also inhibits endocytic sorting, indicating a noncanonical role for a G protein-coupled receptor. Prazosin is a powerful tool for rapid and reversible perturbation of endocytic dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The cytotoxicity of the α1-adrenoceptor antagonist prazosin is linked to an endocytotic mechanism equivalent to transport-P.Toxicology. 2015 Dec 2;338:17-29. doi: 10.1016/j.tox.2015.09.008. Epub 2015 Oct 9. Toxicology. 2015. PMID: 26449523 Free PMC article.
-
APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments.J Cell Biol. 2015 Oct 12;211(1):123-44. doi: 10.1083/jcb.201311117. J Cell Biol. 2015. PMID: 26459602 Free PMC article.
-
NECAP2 controls clathrin coat recruitment to early endosomes for fast endocytic recycling.J Cell Sci. 2016 Jul 1;129(13):2625-37. doi: 10.1242/jcs.173708. Epub 2016 May 20. J Cell Sci. 2016. PMID: 27206861
-
Integration of the Endocytic System into the Network of Cellular Functions.Prog Mol Subcell Biol. 2018;57:39-63. doi: 10.1007/978-3-319-96704-2_2. Prog Mol Subcell Biol. 2018. PMID: 30097771 Review.
-
Endocytosis and Endosomal Trafficking in Plants.Annu Rev Plant Biol. 2016 Apr 29;67:309-35. doi: 10.1146/annurev-arplant-043015-112242. Annu Rev Plant Biol. 2016. PMID: 27128466 Review.
Cited by
-
Prazosin induced lysosomal tubulation interferes with cytokinesis and the endocytic sorting of the tumour antigen CD98hc.Biochim Biophys Acta Mol Cell Res. 2018 Sep;1865(9):1211-1229. doi: 10.1016/j.bbamcr.2018.06.006. Epub 2018 Jun 15. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29909287 Free PMC article.
-
The cytotoxicity of the α1-adrenoceptor antagonist prazosin is linked to an endocytotic mechanism equivalent to transport-P.Toxicology. 2015 Dec 2;338:17-29. doi: 10.1016/j.tox.2015.09.008. Epub 2015 Oct 9. Toxicology. 2015. PMID: 26449523 Free PMC article.
-
G protein-coupled receptors participate in cytokinesis.Cytoskeleton (Hoboken). 2012 Oct;69(10):810-8. doi: 10.1002/cm.21055. Epub 2012 Aug 28. Cytoskeleton (Hoboken). 2012. PMID: 22888021 Free PMC article.
-
Ammonia Induces Autophagy through Dopamine Receptor D3 and MTOR.PLoS One. 2016 Apr 14;11(4):e0153526. doi: 10.1371/journal.pone.0153526. eCollection 2016. PLoS One. 2016. PMID: 27077655 Free PMC article.
-
Dopamine Receptor Subtypes Differentially Regulate Autophagy.Int J Mol Sci. 2018 May 22;19(5):1540. doi: 10.3390/ijms19051540. Int J Mol Sci. 2018. PMID: 29786666 Free PMC article.
References
-
- Lappano R, Maggiolini M. G protein-coupled receptors: Novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. - PubMed
-
- Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol. 2011;23:393–403. - PubMed
-
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. - PubMed
-
- Cavalli V, Corti M, Gruenberg J. Endocytosis and signaling cascades: A close encounter. FEBS Lett. 2001;498:190–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

