Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor
- PMID: 22802634
- PMCID: PMC3411987
- DOI: 10.1073/pnas.1206643109
Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor
Abstract
FSH, a glycoprotein hormone, and the FSH receptor (FSHR), a G protein-coupled receptor, play central roles in human reproduction. We report the crystal structure of FSH in complex with the entire extracellular domain of FSHR (FSHR(ED)), including the enigmatic hinge region that is responsible for signal specificity. Surprisingly, the hinge region does not form a separate structural unit as widely anticipated but is part of the integral structure of FSHR(ED). In addition to the known hormone-binding site, FSHR(ED) provides interaction sites with the hormone: a sulfotyrosine (sTyr) site in the hinge region consistent with previous studies and a potential exosite resulting from putative receptor trimerization. Our structure, in comparison to others, suggests FSHR interacts with its ligand in two steps: ligand recruitment followed by sTyr recognition. FSH first binds to the high-affinity hormone-binding subdomain of FSHR and reshapes the ligand conformation to form a sTyr-binding pocket. FSHR then inserts its sTyr (i.e., sulfated Tyr335) into the FSH nascent pocket, eventually leading to receptor activation.
Conflict of interest statement
Conflict of interest statement: D.F., V.S., H.N.Y., S.A., and X.J. are employees of EMD Serono, Inc., an affiliate of Merck KGaA, Germany, whose commercial products include FSH.
Figures
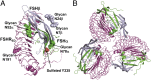




Similar articles
-
Structural biology of glycoprotein hormones and their receptors: insights to signaling.Mol Cell Endocrinol. 2014 Jan 25;382(1):424-451. doi: 10.1016/j.mce.2013.08.021. Epub 2013 Aug 31. Mol Cell Endocrinol. 2014. PMID: 24001578 Review.
-
Assembly and structural characterization of an authentic complex between human follicle stimulating hormone and a hormone-binding ectodomain of its receptor.Mol Cell Endocrinol. 2007 Jan 2;260-262:73-82. doi: 10.1016/j.mce.2005.12.055. Epub 2006 Oct 12. Mol Cell Endocrinol. 2007. PMID: 17045735 Free PMC article.
-
Structural determinants in the second intracellular loop of the human follicle-stimulating hormone receptor are involved in G(s) protein activation.Mol Cell Endocrinol. 2002 Mar 28;189(1-2):157-68. doi: 10.1016/s0303-7207(01)00720-1. Mol Cell Endocrinol. 2002. PMID: 12039074
-
FSH and TSH binding to their respective receptors: similarities, differences and implication for glycoprotein hormone specificity.J Mol Endocrinol. 2008 Sep;41(3):145-64. doi: 10.1677/JME-08-0040. Epub 2008 Jul 7. J Mol Endocrinol. 2008. PMID: 18606720
-
Hormone binding to the follicle-stimulating hormone receptor--crystal clear!Exp Clin Endocrinol Diabetes. 2005 May;113(5):245-7. doi: 10.1055/s-2005-865679. Exp Clin Endocrinol Diabetes. 2005. PMID: 15926107 Review.
Cited by
-
Platelet-derived growth factors and their receptors: structural and functional perspectives.Biochim Biophys Acta. 2013 Oct;1834(10):2176-86. doi: 10.1016/j.bbapap.2012.10.015. Epub 2012 Nov 5. Biochim Biophys Acta. 2013. PMID: 23137658 Free PMC article. Review.
-
The Effect of Stimulation Protocols (GnRH Agonist vs. Antagonist) on the Activity of mTOR and Hippo Pathways of Ovarian Granulosa Cells and Its Potential Correlation with the Outcomes of In Vitro Fertilization: A Hypothesis.J Clin Med. 2022 Oct 18;11(20):6131. doi: 10.3390/jcm11206131. J Clin Med. 2022. PMID: 36294452 Free PMC article.
-
Identification and Relative Quantification of hFSH Glycoforms in Women's Sera via MS-PRM-Based Approach.Pharmaceutics. 2021 May 27;13(6):798. doi: 10.3390/pharmaceutics13060798. Pharmaceutics. 2021. PMID: 34071747 Free PMC article.
-
Identification and characterization of novel compound heterozygous variants in FSHR causing primary ovarian insufficiency with resistant ovary syndrome.Front Endocrinol (Lausanne). 2023 Jan 10;13:1013894. doi: 10.3389/fendo.2022.1013894. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36704038 Free PMC article.
-
Structure and function of LGR5: an enigmatic G-protein coupled receptor marking stem cells.Protein Sci. 2014 May;23(5):551-65. doi: 10.1002/pro.2446. Epub 2014 Mar 19. Protein Sci. 2014. PMID: 24677446 Free PMC article. Review.
References
-
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. - PubMed
-
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. - PubMed
-
- Ulloa-Aguirre A, Zariñán T, Pasapera AM, Casas-González P, Dias JA. Multiple facets of follicle-stimulating hormone receptor function. Endocrine. 2007;32:251–263. - PubMed
-
- Tao YX, Segaloff DL. Follicle stimulating hormone receptor mutations and reproductive disorders. Prog Mol Biol Transl Sci. 2009;89:115–131. - PubMed
-
- Wu H, Lustbader JW, Liu Y, Canfield RE, Hendrickson WA. Structure of human chorionic gonadotropin at 2.6 A resolution from MAD analysis of the selenomethionyl protein. Structure. 1994;2:545–558. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

