E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch
- PMID: 22802638
- PMCID: PMC3411997
- DOI: 10.1073/pnas.1204390109
E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch
Erratum in
- Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):19034
Abstract
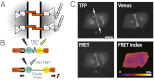
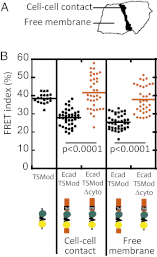
Classical cadherins are transmembrane proteins at the core of intercellular adhesion complexes in cohesive metazoan tissues. The extracellular domain of classical cadherins forms intercellular bonds with cadherins on neighboring cells, whereas the cytoplasmic domain recruits catenins, which in turn associate with additional cytoskeleton binding and regulatory proteins. Cadherin/catenin complexes are hypothesized to play a role in the transduction of mechanical forces that shape cells and tissues during development, regeneration, and disease. Whether mechanical forces are transduced directly through cadherins is unknown. To address this question, we used a Förster resonance energy transfer (FRET)-based molecular tension sensor to test the origin and magnitude of tensile forces transmitted through the cytoplasmic domain of E-cadherin in epithelial cells. We show that the actomyosin cytoskeleton exerts pN-tensile force on E-cadherin, and that this tension requires the catenin-binding domain of E-cadherin and αE-catenin. Surprisingly, the actomyosin cytoskeleton constitutively exerts tension on E-cadherin at the plasma membrane regardless of whether or not E-cadherin is recruited to cell-cell contacts, although tension is further increased at cell-cell contacts when adhering cells are stretched. Our findings thus point to a constitutive role of E-cadherin in transducing mechanical forces between the actomyosin cytoskeleton and the plasma membrane, not only at cell-cell junctions but throughout the cell surface.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Mechanobiology: A measure of molecular muscle.Nature. 2017 Apr 12;544(7649):255-257. doi: 10.1038/544255a. Nature. 2017. PMID: 28406209 No abstract available.
References
-
- Keller R, Shook D, Skoglund P. The forces that shape embryos: Physical aspects of convergent extension by cell intercalation. Phys Biol. 2008;5:015007. - PubMed
-
- Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. - PubMed
-
- Beloussov LV, Dorfman JG, Cherdantzev VG. Mechanical stresses and morphological patterns in amphibian embryos. J Embryol Exp Morphol. 1975;34:559–574. - PubMed
-
- Farge E. Mechanotransduction in development. Curr Top Dev Biol. 2011;95:243–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

