Early, nonciliary role for microtubule proteins in left-right patterning is conserved across kingdoms
- PMID: 22802643
- PMCID: PMC3412009
- DOI: 10.1073/pnas.1202659109
Early, nonciliary role for microtubule proteins in left-right patterning is conserved across kingdoms
Abstract
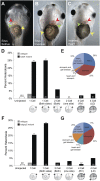
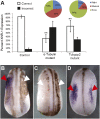
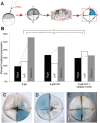
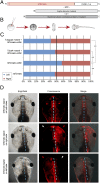
Many types of embryos' bodyplans exhibit consistently oriented laterality of the heart, viscera, and brain. Errors of left-right patterning present an important class of human birth defects, and considerable controversy exists about the nature and evolutionary conservation of the molecular mechanisms that allow embryos to reliably orient the left-right axis. Here we show that the same mutations in the cytoskeletal protein tubulin that alter asymmetry in plants also affect very early steps of left-right patterning in nematode and frog embryos, as well as chirality of human cells in culture. In the frog embryo, tubulin α and tubulin γ-associated proteins are required for the differential distribution of maternal proteins to the left or right blastomere at the first cell division. Our data reveal a remarkable molecular conservation of mechanisms initiating left-right asymmetry. The origin of laterality is cytoplasmic, ancient, and highly conserved across kingdoms, a fundamental feature of the cytoskeleton that underlies chirality in cells and multicellular organisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Conserved roles for cytoskeletal components in determining laterality.Integr Biol (Camb). 2016 Mar 14;8(3):267-86. doi: 10.1039/c5ib00281h. Integr Biol (Camb). 2016. PMID: 26928161 Free PMC article. Review.
-
Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry.Dev Dyn. 2005 Sep;234(1):176-89. doi: 10.1002/dvdy.20509. Dev Dyn. 2005. PMID: 16059906
-
Serotonin has early, cilia-independent roles in Xenopus left-right patterning.Dis Model Mech. 2013 Jan;6(1):261-8. doi: 10.1242/dmm.010256. Epub 2012 Aug 16. Dis Model Mech. 2013. PMID: 22899856 Free PMC article.
-
It's never too early to get it Right: A conserved role for the cytoskeleton in left-right asymmetry.Commun Integr Biol. 2013 Nov 1;6(6):e27155. doi: 10.4161/cib.27155. Epub 2013 Nov 14. Commun Integr Biol. 2013. PMID: 24505508 Free PMC article.
-
From cytoskeletal dynamics to organ asymmetry: a nonlinear, regulative pathway underlies left-right patterning.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150409. doi: 10.1098/rstb.2015.0409. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821521 Free PMC article. Review.
Cited by
-
Cilia in vertebrate left-right patterning.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150410. doi: 10.1098/rstb.2015.0410. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821522 Free PMC article. Review.
-
Epithelial Cell Chirality Revealed by Three-Dimensional Spontaneous Rotation.Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12188-12193. doi: 10.1073/pnas.1805932115. Epub 2018 Nov 14. Proc Natl Acad Sci U S A. 2018. PMID: 30429314 Free PMC article.
-
Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo.Mol Biol Cell. 2014 Dec 1;25(24):3835-50. doi: 10.1091/mbc.E13-12-0708. Mol Biol Cell. 2014. PMID: 25425556 Free PMC article.
-
Exome-wide analysis implicates rare protein-altering variants in human handedness.Nat Commun. 2024 Apr 2;15(1):2632. doi: 10.1038/s41467-024-46277-w. Nat Commun. 2024. PMID: 38565598 Free PMC article.
-
The genetic architecture of structural left-right asymmetry of the human brain.Nat Hum Behav. 2021 Sep;5(9):1226-1239. doi: 10.1038/s41562-021-01069-w. Epub 2021 Mar 15. Nat Hum Behav. 2021. PMID: 33723403 Free PMC article.
References
-
- Peeters H, Devriendt K. Human laterality disorders. Eur J Med Genet. 2006;49:349–362. - PubMed
-
- Tabin C. Do we know anything about how left-right asymmetry is first established in the vertebrate embryo? J Mol Histol. 2005;36:317–323. - PubMed
-
- Wolpert L. Diffusible gradients are out - an interview with Lewis Wolpert. Interviewed by Richardson, Michael K. Int J Dev Biol. 2009;53:659–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

