Fighting malaria with engineered symbiotic bacteria from vector mosquitoes
- PMID: 22802646
- PMCID: PMC3412027
- DOI: 10.1073/pnas.1204158109
Fighting malaria with engineered symbiotic bacteria from vector mosquitoes
Abstract
The most vulnerable stages of Plasmodium development occur in the lumen of the mosquito midgut, a compartment shared with symbiotic bacteria. Here, we describe a strategy that uses symbiotic bacteria to deliver antimalaria effector molecules to the midgut lumen, thus rendering host mosquitoes refractory to malaria infection. The Escherichia coli hemolysin A secretion system was used to promote the secretion of a variety of anti-Plasmodium effector proteins by Pantoea agglomerans, a common mosquito symbiotic bacterium. These engineered P. agglomerans strains inhibited development of the human malaria parasite Plasmodium falciparum and rodent malaria parasite Plasmodium berghei by up to 98%. Significantly, the proportion of mosquitoes carrying parasites (prevalence) decreased by up to 84% for two of the effector molecules, scorpine, a potent antiplasmodial peptide and (EPIP)(4), four copies of Plasmodium enolase-plasminogen interaction peptide that prevents plasminogen binding to the ookinete surface. We demonstrate the use of an engineered symbiotic bacterium to interfere with the development of P. falciparum in the mosquito. These findings provide the foundation for the use of genetically modified symbiotic bacteria as a powerful tool to combat malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
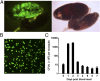
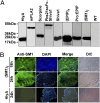
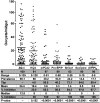
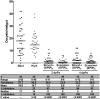

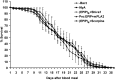
Comment in
-
Antimicrobials: Arming symbionts with antimalarials.Nat Rev Microbiol. 2012 Sep;10(9):594-5. doi: 10.1038/nrmicro2860. Epub 2012 Aug 6. Nat Rev Microbiol. 2012. PMID: 22864263 No abstract available.
References
-
- Murray CJ, et al. Global malaria mortality between 1980 and 2010: A systematic analysis. Lancet. 2012;379:413–431. - PubMed
-
- Enayati A, Hemingway J. Malaria management: Past, present, and future. Annu Rev Entomol. 2010;55:569–591. - PubMed
-
- Trape JF, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: A longitudinal study. Lancet Infect Dis. 2011;11:925–932. - PubMed
-
- Ghosh A, Edwards MJ, Jacobs-Lorena M. The journey of the malaria parasite in the mosquito: Hopes for the new century. Parasitol Today. 2000;16:196–201. - PubMed
-
- Whitten MMA, Shiao SH, Levashina EA. Mosquito midguts and malaria: Cell biology, compartmentalization and immunology. Parasite Immunol. 2006;28:121–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

