Crystal structure of IgE bound to its B-cell receptor CD23 reveals a mechanism of reciprocal allosteric inhibition with high affinity receptor FcεRI
- PMID: 22802656
- PMCID: PMC3412039
- DOI: 10.1073/pnas.1207278109
Crystal structure of IgE bound to its B-cell receptor CD23 reveals a mechanism of reciprocal allosteric inhibition with high affinity receptor FcεRI
Abstract
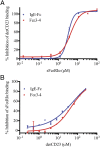
The role of IgE in allergic disease mechanisms is performed principally through its interactions with two receptors, FcεRI on mast cells and basophils, and CD23 (FcεRII) on B cells. The former mediates allergic hypersensitivity, the latter regulates IgE levels, and both receptors, also expressed on antigen-presenting cells, contribute to allergen uptake and presentation to the immune system. We have solved the crystal structure of the soluble lectin-like "head" domain of CD23 (derCD23) bound to a subfragment of IgE-Fc consisting of the dimer of Cε3 and Cε4 domains (Fcε3-4). One CD23 head binds to each heavy chain at the interface between the two domains, explaining the known 2:1 stoichiometry and suggesting mechanisms for cross-linking membrane-bound trimeric CD23 by IgE, or membrane IgE by soluble trimeric forms of CD23, both of which may contribute to the regulation of IgE synthesis by B cells. The two symmetrically located binding sites are distant from the single FcεRI binding site, which lies at the opposite ends of the Cε3 domains. Structural comparisons with both free IgE-Fc and its FcεRI complex reveal not only that the conformational changes in IgE-Fc required for CD23 binding are incompatible with FcεRI binding, but also that the converse is true. The two binding sites are allosterically linked. We demonstrate experimentally the reciprocal inhibition of CD23 and FcεRI binding in solution and suggest that the mutual exclusion of receptor binding allows IgE to function independently through its two receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. - PubMed
-
- Garman SC, Kinet J-P, Jardetzky TS. Crystal structure of the human high-affinity IgE receptor. Cell. 1998;95:951–961. - PubMed
-
- Shi J, et al. Interaction of the low-affinity receptor CD23/Fc epsilonRII lectin domain with the Fc ε3-4 fragment of human immunoglobulin E. Biochemistry. 1997;36:2112–2122. - PubMed
-
- McDonnell JM, et al. The structure of the IgE Cepsilon2 domain and its role in stabilizing the complex with its high-affinity receptor FcepsilonRIalpha. Nat Struct Biol. 2001;8:437–441. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

