Tat-Mediated p66shc Transduction Decreased Phosphorylation of Endothelial Nitric Oxide Synthase in Endothelial Cells
- PMID: 22802702
- PMCID: PMC3394923
- DOI: 10.4196/kjpp.2012.16.3.199
Tat-Mediated p66shc Transduction Decreased Phosphorylation of Endothelial Nitric Oxide Synthase in Endothelial Cells
Abstract
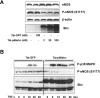
We evaluated the role of Tat-mediated p66shc transduction on the activation of endothelial nitric oxide synthase in cultured mouse endothelial cells. To construct the Tat-p66shc fusion protein, human full length p66shc cDNA was fused with the Tat-protein transduction domain. Transduction of TAT-p66shc showed a concentration- and time-dependent manner in endothelial cells. Tat-mediated p66shc transduction showed increased hydrogen peroxide and superoxide production, compared with Tat-p66shc (S/A), serine 36 residue mutant of p66shc. Tat-mediated p66shc transduction decreased endothelial nitric oxide synthase phosphorylation in endothelial cells. Furthermore, Tat-mediated p66shc transduction augmented TNF-α-induced p38 MAPK phosphorylation in endothelial cells. These results suggest that Tat-mediated p66shc transduction efficiently inhibited endothelial nitric oxide synthase phosphorylation in endothelial cells.
Keywords: Endothelial cells; Endothelial nitric oxide synthase; Superoxide; Tat-mediated transduction; p66shc.
Figures



References
-
- Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. - PubMed
-
- Molavi B, Mehta JL. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr Opin Cardiol. 2004;19:488–493. - PubMed
-
- Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. - PubMed
-
- Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003;100:2112–2116. - PMC - PubMed

