Regulation of Matrix Remodeling by Peroxisome Proliferator-Activated Receptor-γ: A Novel Link Between Metabolism and Fibrogenesis
- PMID: 22802908
- PMCID: PMC3396343
- DOI: 10.2174/1874312901206010103
Regulation of Matrix Remodeling by Peroxisome Proliferator-Activated Receptor-γ: A Novel Link Between Metabolism and Fibrogenesis
Abstract
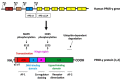
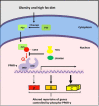
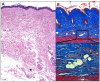
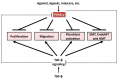
The intractable process of fibrosis underlies the pathogenesis of systemic sclerosis (SSc) and other diseases, and in aggregate contributes to 45% of deaths worldwide. Because currently there is no effective anti-fibrotic therapy, a better understanding of the pathways and cellular differentiation programs underlying fibrosis are needed. Emerging evidence points to a fundamental role of the nuclear hormone receptor peroxisome proliferator activated receptor-γ (PPAR-γ) in modulating fibrogenesis. While PPAR-γ has long been known to be important in lipid metabolism and in glucose homeostasis, its role in regulating mesenchymal cell biology and its association with pathological fibrosis had not been appreciated until recently. This article highlights recent studies revealing a consistent association of fibrosis with aberrant PPAR-γ expression and activity in various forms of human fibrosis and in rodent models, and reviews studies linking genetic manipulation of the PPAR-γ pathway in rodents and fibrosis. We survey the broad range of anti-fibrotic activities associated with PPAR-γ and the underlying mechanisms. We also summarize the emerging data linking PPAR-γ dysfunction and pulmonary arterial hypertension (PAH), which together with fibrosis is responsible for the mortality in patients in SSc. Finally, we consider current and potential future strategies for targeting PPAR-γ activity or expression as a therapy for controlling fibrosis.
Keywords: PPAR-γ; SPPARM; TGF-β.; systemic sclerosis.
Figures

References
-
- Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9(2):136–43. - PubMed
-
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–56. - PubMed
-
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123(6):993–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
