Glucocerebrosidase mutations alter the endoplasmic reticulum and lysosomes in Lewy body disease
- PMID: 22803570
- PMCID: PMC3494984
- DOI: 10.1111/j.1471-4159.2012.07879.x
Glucocerebrosidase mutations alter the endoplasmic reticulum and lysosomes in Lewy body disease
Abstract
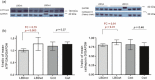
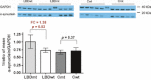
Lewy body disease (LBD) development is enhanced by mutations in the GBA gene coding for glucocerebrosidase (GCase). The mechanism of this association is thought to involve an abnormal lysosomal system and we therefore sought to evaluate if lysosomal changes contribute to the pathogenesis of idiopathic LBD. Analysis of post-mortem frontal cortex tissue from 7 GBA mutation carriers with LBD, 5 GBA mutation carriers with no signs of neurological disease and human neural stem cells exposed to a GCase inhibitor was used to determine how GBA mutation contributes to LBD. GBA mutation carriers demonstrated a significantly reduced level of GCase protein and enzyme activity and retention of glucocerebrosidase isoforms within the endoplasmic reticulum (ER). This was associated with enhanced expression of the lysosomal markers LAMP1 and LAMP2, though the expression of ATP13A2 and Cathepsin D was reduced, along with the decreased activity of Cathepsin D. The ER unfolded protein response (UPR) regulator BiP/GRP78 was reduced by GBA mutation and this was a general phenomenon in LBD. Despite elevation of GRP94 in LBD, individuals with GBA mutations showed reduced GRP94 expression, suggesting an inadequate UPR. Finally, human neural stem cell cultures showed that inhibition of GCase causes acute reduction of BiP, indicating that the UPR is affected by reduced glucocerebrosidase activity. The results indicate that mutation in GBA leads to additional lysosomal abnormalities, enhanced by an impaired UPR, potentially causing α-synuclein accumulation.
© 2012 The Authors Journal of Neurochemistry © 2012 International Society for Neurochemistry.
Figures






References
-
- Barranger JA, Ginns EI. The Metabolic Basis of Inherited Disease. New York: McGraw-Hill Information Services Company; 1989.
-
- Burnstein RM, Foltynie T, He X, Menon DK, Svendsen CN, Caldwell MA. Differentiation and migration of long term expanded human neural progenitors in a partial lesion model of Parkinson’s disease. Int. J. Biochem. Cell Biol. 2004;36:702–713. - PubMed
-
- Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson’s disease? Neurobiol. Dis. 2007;25:134–149. - PubMed
-
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol. Dis. 2009;35:385–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

