Novel B(12)-dependent acyl-CoA mutases and their biotechnological potential
- PMID: 22803641
- PMCID: PMC3448797
- DOI: 10.1021/bi300827v
Novel B(12)-dependent acyl-CoA mutases and their biotechnological potential
Abstract
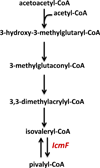
The recent spate of discoveries of novel acyl-CoA mutases has engendered a growing appreciation for the diversity of 5'-deoxyadenosylcobalamin-dependent rearrangement reactions. The prototype of the reaction catalyzed by these enzymes is the 1,2 interchange of a hydrogen atom with a thioester group leading to a change in the degree of carbon skeleton branching. These enzymes are predicted to share common architectural elements: a Rossman fold and a triose phosphate isomerase (TIM)-barrel domain for binding cofactor and substrate, respectively. Within this family, methylmalonyl-CoA mutase (MCM) is the best studied and is the only member found in organisms ranging from bacteria to man. MCM interconverts (2R)-methylmalonyl-CoA and succinyl-CoA. The more recently discovered family members include isobutyryl-CoA mutase (ICM), which interconverts isobutyryl-CoA and n-butyryl-CoA; ethylmalonyl-CoA mutase, which interconverts (2R)-ethylmalonyl-CoA and (2S)-methylsuccinyl-CoA; and 2-hydroxyisobutyryl-CoA mutase, which interconverts 2-hydroxyisobutyryl-CoA and (3S)-hydroxybutyryl-CoA. A variant in which the two subunits of ICM are fused to a G-protein chaperone, IcmF, has been described recently. In addition to its ICM activity, IcmF also catalyzes the interconversion of isovaleryl-CoA and pivalyl-CoA. This review focuses on the involvement of acyl-CoA mutases in central carbon and secondary bacterial metabolism and on their biotechnological potential for applications ranging from bioremediation to stereospecific synthesis of C2-C5 carboxylic acids and alcohols, and for production of potential commodity and specialty chemicals.
Figures





References
-
- Banerjee R. Radical carbon skeleton rearrangements: catalysis by coenzyme B12-dependent mutases. Chem Rev. 2003;103:2083–2094. - PubMed
-
- Frey PA. Radical mechanisms of enzymatic catalysis. Ann Rev Biochem. 2001;70:121–148. - PubMed
-
- Dowling DP, Croft AK, Drennan CL. Radical Use of Rossmann and TIM Barrel Architectures for Controlling Coenzyme B12Chemistry. Annu Rev Biophys. 2012;41:403–427. - PubMed
-
- Hay BP, Finke RG. Thermolysis of the Co-C bond in adenosylcorrins. 3. Quantifiation of the axial base effect in adenosylcobalamin by the synthesis and thermolysis of axial base-free adenosylcobinamide. Insights into the energetics of enzyme-assisted cobalt-carbon bond homolysis. J. Am. Chem. Soc. 1987;109:8012–8018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

