Double-blind, randomized, placebo-controlled study of safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-683, an investigational metastin analogue in healthy men
- PMID: 22803642
- PMCID: PMC3579253
- DOI: 10.1111/j.1365-2125.2012.04385.x
Double-blind, randomized, placebo-controlled study of safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-683, an investigational metastin analogue in healthy men
Abstract
Aims: Two randomized, double-blind, placebo-controlled studies were performed to characterize the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of the investigational metastin analogue, TAK-683, in healthy men.
Methods: We first investigated a single subcutaneous (s.c.) dose of TAK-683 (0.01-2.0 mg) in 60 subjects (TAK-683, n = 42; placebo, n = 18). We then assessed a single s.c. bolus of 0.03-1.0 mg TAK-683 on day 1, followed by a 0.01-2.0 mg day(-1) continuous infusion on days 2-13, to simulate a depot formulation, in 30 subjects (TAK-683, n = 25; placebo, n = 5) for 14 days.
Results: TAK-683 was well tolerated up to a dose of 2.0 mg day(-1) by continuous s.c. infusion for 14 days. Adverse events were similar between TAK-683 and placebo subjects at all dose levels. TAK-683 plasma concentrations generally increased in proportion to dose with single and continuous dosing, with steady-state concentrations achieved by day 2 of continuous dosing. TAK-683 at 2.0 mg day(-1) suppressed testosterone below castration level (<50 ng dl(-1)) in four of five subjects by day 7 of continuous dosing. Luteinizing hormone and follicle stimulating hormone concentrations were suppressed with TAK-683 continuous dosing compared with placebo by up to 70 and 43%, respectively, but this was not consistently dose-dependent.
Conclusions: In healthy men, s.c. administration of TAK-683 was well tolerated at all dose levels. The PK profile of TAK-683 was favourable, and TAK-683 suppressed testosterone profoundly during continuous dosing. Further investigation of metastin analogues is warranted for the treatment of castration-resistant prostate cancer.
© 2012 Takeda Global Research & Development Centre (Europe) Ltd, London, UK. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
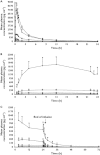
 , TAK-683 0.01 mg day−1 (Cohort 1).
, TAK-683 0.01 mg day−1 (Cohort 1).  , TAK-683 0.03 mg day−1 (Cohort 2).
, TAK-683 0.03 mg day−1 (Cohort 2).  , TAK-683 0.1 mg day−1 (Cohort 3).
, TAK-683 0.1 mg day−1 (Cohort 3).  , TAK-683 0.3 mg day−1 (Cohort 4).
, TAK-683 0.3 mg day−1 (Cohort 4).  , TAK-683 2.0 mg day−1 (Cohort 5)
, TAK-683 2.0 mg day−1 (Cohort 5)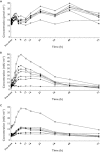
 , placebo.
, placebo.  , TAK-683 0.01 mg.
, TAK-683 0.01 mg.  , TAK-683 0.03 mg.
, TAK-683 0.03 mg.  , TAK-683 0.1 mg.
, TAK-683 0.1 mg.  , TAK-683 0.3 mg.
, TAK-683 0.3 mg.  , TAK-683 1.0 mg.
, TAK-683 1.0 mg.  , TAK-683 2.0 mg
, TAK-683 2.0 mg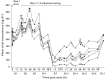
 , 0.01 mg kg–1.
, 0.01 mg kg–1.  , 0.03 mg kg–1.
, 0.03 mg kg–1.  , 0.10 mg kg–1.
, 0.10 mg kg–1.  , 0.30 mg kg–1.
, 0.30 mg kg–1.  , 2.00 mg kg–1
, 2.00 mg kg–1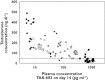
Similar articles
-
Sustained exposure to the investigational Kisspeptin analog, TAK-448, down-regulates testosterone into the castration range in healthy males and in patients with prostate cancer: results from two phase 1 studies.J Clin Endocrinol Metab. 2014 Aug;99(8):E1445-53. doi: 10.1210/jc.2013-4236. Epub 2014 Apr 24. J Clin Endocrinol Metab. 2014. PMID: 24762108 Clinical Trial.
-
Pharmacologic profiles of investigational kisspeptin/metastin analogues, TAK-448 and TAK-683, in adult male rats in comparison to the GnRH analogue leuprolide.Eur J Pharmacol. 2014 Jul 15;735:77-85. doi: 10.1016/j.ejphar.2014.03.058. Epub 2014 Apr 18. Eur J Pharmacol. 2014. PMID: 24747751
-
Medical Castration Using the Investigational Oral GnRH Antagonist TAK-385 (Relugolix): Phase 1 Study in Healthy Males.J Clin Endocrinol Metab. 2015 Dec;100(12):4579-87. doi: 10.1210/jc.2015-2770. Epub 2015 Oct 26. J Clin Endocrinol Metab. 2015. PMID: 26502357 Free PMC article. Clinical Trial.
-
Safety, tolerability, pharmacokinetics and pharmacodynamics of the anti-CD38 cytolytic antibody TAK-079 in healthy subjects.Br J Clin Pharmacol. 2020 Jul;86(7):1314-1325. doi: 10.1111/bcp.14241. Epub 2020 Feb 22. Br J Clin Pharmacol. 2020. PMID: 32045493 Free PMC article. Clinical Trial.
-
Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects.Aliment Pharmacol Ther. 2015 Apr;41(7):636-48. doi: 10.1111/apt.13121. Epub 2015 Feb 23. Aliment Pharmacol Ther. 2015. PMID: 25707624 Free PMC article. Clinical Trial.
Cited by
-
Kisspeptin receptor agonist (FTM080) increased plasma concentrations of luteinizing hormone in anestrous ewes.PeerJ. 2015 Nov 5;3:e1382. doi: 10.7717/peerj.1382. eCollection 2015. PeerJ. 2015. PMID: 26587345 Free PMC article.
-
The kisspeptin-GnRH pathway in human reproductive health and disease.Hum Reprod Update. 2014 Jul-Aug;20(4):485-500. doi: 10.1093/humupd/dmu009. Epub 2014 Mar 9. Hum Reprod Update. 2014. PMID: 24615662 Free PMC article. Review.
-
A synthetic kisspeptin analog that triggers ovulation and advances puberty.Sci Rep. 2016 Jun 1;6:26908. doi: 10.1038/srep26908. Sci Rep. 2016. PMID: 27245315 Free PMC article.
-
Advances in clinical applications of kisspeptin-GnRH pathway in female reproduction.Reprod Biol Endocrinol. 2022 May 23;20(1):81. doi: 10.1186/s12958-022-00953-y. Reprod Biol Endocrinol. 2022. PMID: 35606759 Free PMC article. Review.
-
Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour.Nat Rev Endocrinol. 2021 Feb;17(2):97-113. doi: 10.1038/s41574-020-00438-1. Epub 2020 Dec 3. Nat Rev Endocrinol. 2021. PMID: 33273729 Review.
References
-
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. - PubMed
-
- Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–2387. - PubMed
-
- Mitchell DC, Abdelrahim M, Weng J, Stafford LJ, Safe S, Bar-Eli M, Liu M. Regulation of KiSS-1 metastasis suppressor gene expression in breast cancer cells by direct interaction of transcription factors activator protein-2alpha and specificity protein-1. J Biol Chem. 2006;281:51–58. - PubMed
-
- Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, Ito D, Peiper SC, Broach JR, Oishi S, Niida A, Fujii N, Imamura M. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun. 2004;315:85–92. - PubMed
-
- Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:1379–1383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

