Double-blind, randomized, placebo-controlled study of safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-683, an investigational metastin analogue in healthy men
- PMID: 22803642
- PMCID: PMC3579253
- DOI: 10.1111/j.1365-2125.2012.04385.x
Double-blind, randomized, placebo-controlled study of safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-683, an investigational metastin analogue in healthy men
Abstract
Aims: Two randomized, double-blind, placebo-controlled studies were performed to characterize the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of the investigational metastin analogue, TAK-683, in healthy men.
Methods: We first investigated a single subcutaneous (s.c.) dose of TAK-683 (0.01-2.0 mg) in 60 subjects (TAK-683, n = 42; placebo, n = 18). We then assessed a single s.c. bolus of 0.03-1.0 mg TAK-683 on day 1, followed by a 0.01-2.0 mg day(-1) continuous infusion on days 2-13, to simulate a depot formulation, in 30 subjects (TAK-683, n = 25; placebo, n = 5) for 14 days.
Results: TAK-683 was well tolerated up to a dose of 2.0 mg day(-1) by continuous s.c. infusion for 14 days. Adverse events were similar between TAK-683 and placebo subjects at all dose levels. TAK-683 plasma concentrations generally increased in proportion to dose with single and continuous dosing, with steady-state concentrations achieved by day 2 of continuous dosing. TAK-683 at 2.0 mg day(-1) suppressed testosterone below castration level (<50 ng dl(-1)) in four of five subjects by day 7 of continuous dosing. Luteinizing hormone and follicle stimulating hormone concentrations were suppressed with TAK-683 continuous dosing compared with placebo by up to 70 and 43%, respectively, but this was not consistently dose-dependent.
Conclusions: In healthy men, s.c. administration of TAK-683 was well tolerated at all dose levels. The PK profile of TAK-683 was favourable, and TAK-683 suppressed testosterone profoundly during continuous dosing. Further investigation of metastin analogues is warranted for the treatment of castration-resistant prostate cancer.
© 2012 Takeda Global Research & Development Centre (Europe) Ltd, London, UK. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
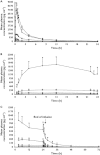
 , TAK-683 0.01 mg day−1 (Cohort 1).
, TAK-683 0.01 mg day−1 (Cohort 1).  , TAK-683 0.03 mg day−1 (Cohort 2).
, TAK-683 0.03 mg day−1 (Cohort 2).  , TAK-683 0.1 mg day−1 (Cohort 3).
, TAK-683 0.1 mg day−1 (Cohort 3).  , TAK-683 0.3 mg day−1 (Cohort 4).
, TAK-683 0.3 mg day−1 (Cohort 4).  , TAK-683 2.0 mg day−1 (Cohort 5)
, TAK-683 2.0 mg day−1 (Cohort 5)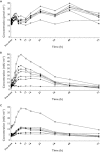
 , placebo.
, placebo.  , TAK-683 0.01 mg.
, TAK-683 0.01 mg.  , TAK-683 0.03 mg.
, TAK-683 0.03 mg.  , TAK-683 0.1 mg.
, TAK-683 0.1 mg.  , TAK-683 0.3 mg.
, TAK-683 0.3 mg.  , TAK-683 1.0 mg.
, TAK-683 1.0 mg.  , TAK-683 2.0 mg
, TAK-683 2.0 mg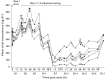
 , 0.01 mg kg–1.
, 0.01 mg kg–1.  , 0.03 mg kg–1.
, 0.03 mg kg–1.  , 0.10 mg kg–1.
, 0.10 mg kg–1.  , 0.30 mg kg–1.
, 0.30 mg kg–1.  , 2.00 mg kg–1
, 2.00 mg kg–1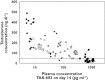
References
-
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. - PubMed
-
- Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–2387. - PubMed
-
- Mitchell DC, Abdelrahim M, Weng J, Stafford LJ, Safe S, Bar-Eli M, Liu M. Regulation of KiSS-1 metastasis suppressor gene expression in breast cancer cells by direct interaction of transcription factors activator protein-2alpha and specificity protein-1. J Biol Chem. 2006;281:51–58. - PubMed
-
- Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, Ito D, Peiper SC, Broach JR, Oishi S, Niida A, Fujii N, Imamura M. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun. 2004;315:85–92. - PubMed
-
- Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:1379–1383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

