Ruminant organotypic brain-slice cultures as a model for the investigation of CNS listeriosis
- PMID: 22804762
- PMCID: PMC3444982
- DOI: 10.1111/j.1365-2613.2012.00821.x
Ruminant organotypic brain-slice cultures as a model for the investigation of CNS listeriosis
Abstract
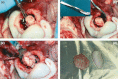
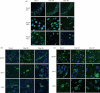
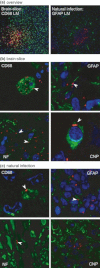
Central nervous system (CNS) infections in ruminant livestock, such as listeriosis, are of major concern for veterinary and public health. To date, no host-specific in vitro models for ruminant CNS infections are available. Here, we established and evaluated the suitability of organotypic brain-slices of ruminant origin as in vitro model to study mechanisms of Listeria monocytogenes CNS infection. Ruminants are frequently affected by fatal listeric rhombencephalitis that closely resembles the same condition occurring in humans. Better insight into host-pathogen interactions in ruminants is therefore of interest, not only from a veterinary but also from a public health perspective. Brains were obtained at the slaughterhouse, and hippocampal and cerebellar brain-slices were cultured up to 49 days. Viability as well as the composition of cell populations was assessed weekly. Viable neurons, astrocytes, microglia and oligodendrocytes were observed up to 49 days in vitro. Slice cultures were infected with L. monocytogenes, and infection kinetics were monitored. Infected brain cells were identified by double immunofluorescence, and results were compared to natural cases of listeric rhombencephalitis. Similar to the natural infection, infected brain-slices showed focal replication of L. monocytogenes and bacteria were predominantly observed in microglia, but also in astrocytes, and associated with axons. These results demonstrate that organotypic brain-slice cultures of bovine origin survive for extended periods and can be infected easily with L. monocytogenes. Therefore, they are a suitable model to study aspects of host-pathogen interaction in listeric encephalitis and potentially in other neuroinfectious diseases.
© 2012 The Authors. International Journal of Experimental Pathology © 2012 International Journal of Experimental Pathology.
Figures



Similar articles
-
Increased spread and replication efficiency of Listeria monocytogenes in organotypic brain-slices is related to multilocus variable number of tandem repeat analysis (MLVA) complex.BMC Microbiol. 2015 Jul 3;15:134. doi: 10.1186/s12866-015-0454-0. BMC Microbiol. 2015. PMID: 26138984 Free PMC article.
-
Infection of murine fetal brain cell cultures with Listeria monocytogenes.Vet Microbiol. 1994 Jul;41(1-2):19-28. doi: 10.1016/0378-1135(94)90132-5. Vet Microbiol. 1994. PMID: 7801522
-
The distribution of E-cadherin expression in listeric rhombencephalitis of ruminants indicates its involvement in Listeria monocytogenes neuroinvasion.Neuropathol Appl Neurobiol. 2011 Dec;37(7):753-67. doi: 10.1111/j.1365-2990.2011.01183.x. Neuropathol Appl Neurobiol. 2011. PMID: 21486315
-
Rhombencephalitis due to Listeria monocytogenes infection with GQ1b antibody positivity and multiple intracranial hemorrhage: a case report and literature review.J Int Med Res. 2021 Apr;49(4):300060521998568. doi: 10.1177/0300060521998568. J Int Med Res. 2021. PMID: 33866842 Free PMC article. Review.
-
Targeting of the central nervous system by Listeria monocytogenes.Virulence. 2012 Mar-Apr;3(2):213-21. doi: 10.4161/viru.19586. Epub 2012 Mar 1. Virulence. 2012. PMID: 22460636 Free PMC article. Review.
Cited by
-
Listeria monocytogenes spreads within the brain by actin-based intra-axonal migration.Infect Immun. 2015 Jun;83(6):2409-19. doi: 10.1128/IAI.00316-15. Epub 2015 Mar 30. Infect Immun. 2015. PMID: 25824833 Free PMC article.
-
Organotypic slice culture based on in ovo electroporation for chicken embryonic central nervous system.J Cell Mol Med. 2019 Mar;23(3):1813-1826. doi: 10.1111/jcmm.14080. Epub 2018 Dec 18. J Cell Mol Med. 2019. PMID: 30565384 Free PMC article.
-
Current methodologies available to evaluate the virulence potential among Listeria monocytogenes clonal complexes.Front Microbiol. 2024 Oct 10;15:1425437. doi: 10.3389/fmicb.2024.1425437. eCollection 2024. Front Microbiol. 2024. PMID: 39493856 Free PMC article. Review.
-
Bovine Brain: An in vitro Translational Model in Developmental Neuroscience and Neurodegenerative Research.Front Pediatr. 2014 Jul 10;2:74. doi: 10.3389/fped.2014.00074. eCollection 2014. Front Pediatr. 2014. PMID: 25072040 Free PMC article. Review.
-
Bovine neutrophil chemotaxis to Listeria monocytogenes in neurolisteriosis depends on microglia-released rather than bacterial factors.J Neuroinflammation. 2022 Dec 16;19(1):304. doi: 10.1186/s12974-022-02653-1. J Neuroinflammation. 2022. PMID: 36527076 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

