Development and evolution of the vertebrate primary mouth
- PMID: 22804777
- PMCID: PMC3552417
- DOI: 10.1111/j.1469-7580.2012.01540.x
Development and evolution of the vertebrate primary mouth
Abstract
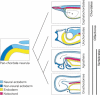
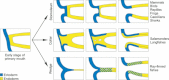
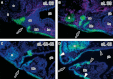
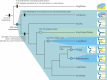
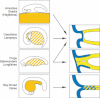
The vertebrate oral region represents a key interface between outer and inner environments, and its structural and functional design is among the limiting factors for survival of its owners. Both formation of the respective oral opening (primary mouth) and establishment of the food-processing apparatus (secondary mouth) require interplay between several embryonic tissues and complex embryonic rearrangements. Although many aspects of the secondary mouth formation, including development of the jaws, teeth or taste buds, are known in considerable detail, general knowledge about primary mouth formation is regrettably low. In this paper, primary mouth formation is reviewed from a comparative point of view in order to reveal its underestimated morphogenetic diversity among, and also within, particular vertebrate clades. In general, three main developmental modes were identified. The most common is characterized by primary mouth formation via a deeply invaginated ectodermal stomodeum and subsequent rupture of the bilaminar oral membrane. However, in salamander, lungfish and also in some frog species, the mouth develops alternatively via stomodeal collar formation contributed both by the ecto- and endoderm. In ray-finned fishes, on the other hand, the mouth forms via an ectoderm wedge and later horizontal detachment of the initially compressed oral epithelia with probably a mixed germ-layer derivation. A very intriguing situation can be seen in agnathan fishes: whereas lampreys develop their primary mouth in a manner similar to the most common gnathostome pattern, hagfishes seem to undergo a unique oropharyngeal morphogenesis when compared with other vertebrates. In discussing the early formative embryonic correlates of primary mouth formation likely to be responsible for evolutionary-developmental modifications of this area, we stress an essential role of four factors: first, positioning and amount of yolk tissue; closely related to, second, endoderm formation during gastrulation, which initiates the process and constrains possible evolutionary changes within this area; third, incipient structure of the stomodeal primordium at the anterior neural plate border, where the ectoderm component of the prospective primary mouth is formed; and fourth, the prime role of Pitx genes for establishment and later morphogenesis of oral region both in vertebrates and non-vertebrate chordates.
© 2012 The Authors. Journal of Anatomy © 2012 Anatomical Society.
Figures



References
-
- Adams AE. An experimental study of the development of the mouth in the amphibian embryo. J Exp Zool. 1924;40:311–379.
-
- Adams AE. Some effects of the removal of endoderm from the mouth region of the early Amblystoma punctatum embryos. J Exp Zool. 1931;58:147–163.
-
- Ahrens K, Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev Biol. 2005;288:40–59. - PubMed
-
- Angotzi AR, Ersland KM, Mungpakdee S, et al. Independent and dynamic reallocation of pitx gene expression during vertebrate evolution, with emphasis on fish pituitary development. Gene. 2008;417:19–26. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

