Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal
- PMID: 22804785
- PMCID: PMC3477306
- DOI: 10.1111/j.1369-1600.2012.00466.x
Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal
Abstract
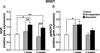
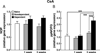
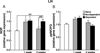
Anxiety is a key consequence of ethanol withdrawal and important risk factor for relapse. The neuropeptide nociceptin/orphanin FQ (N/OFQ) or agonists at this peptide's receptor (NOP) exert anxiolytic-like and antistress actions. N/OFQ dysfunction has been linked to both a high-anxiety behavioral phenotype and excessive ethanol intake. Recent studies suggest a possible link between genetic polymorphisms of the NOP transcript and alcoholism. Thus, in the present study, the effects of intracerebroventricularly administered N/OFQ were tested for modification of anxiety-like behaviors, using the shock-probe defensive burying and elevated plus-maze tests, in ethanol-dependent versus non-dependent rats, 1 and 3 weeks following termination of ethanol exposure. Additionally, prepro-N/OFQ (ppN/OFQ) and NOP receptor gene expression was measured in the central nucleus of the amygdala, in the bed nucleus of the stria terminalis and in the lateral hypothalamus at the same timepoints in separate subjects. One week post-ethanol, N/OFQ dose-dependently attenuated elevated anxiety-like behavior in ethanol-dependent rats and produced anxiolytic-like effects in non-dependent controls in both behavioral tests. However, 3 weeks post-ethanol, N/OFQ altered behavior consistent with anxiogenic-like actions in ethanol-dependent rats but continued to exert anxiolytic-like actions in non-dependent controls. These findings were paralleled by ethanol history-dependent changes of ppN/OFQ and NOP gene expression that showed a distinctive time course in the examined brain structures. The results demonstrate that ethanol dependence and withdrawal are associated with neuroadaptive changes in the N/OFQ-NOP system, suggesting a role of this neuropeptidergic pathway as a therapeutic target for the treatment of alcohol abuse.
© 2012 The Authors, Addiction Biology © 2012 Society for the Study of Addiction.
Conflict of interest statement
Disclosure/Conflict of Interest
None of the authors have any conflicts of interest relating to the work described in this manuscript.
Figures


References
-
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. - PubMed
-
- Calo' G, Rizzi A, Bigoni R, Guerrini R, Salvadori S, Regoli D. Pharmacological profile of nociceptin/orphanin FQ receptors. Clin Exp Pharmacol Physiol. 2002;29:223–228. - PubMed
-
- Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M. Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64-6198. Psychopharmacology. 2002;161:113–119. - PubMed

