Design and rationale of safe pediatric euglycemia after cardiac surgery: a randomized controlled trial of tight glycemic control after pediatric cardiac surgery
- PMID: 22805161
- PMCID: PMC3477238
- DOI: 10.1097/PCC.0b013e31825b549a
Design and rationale of safe pediatric euglycemia after cardiac surgery: a randomized controlled trial of tight glycemic control after pediatric cardiac surgery
Abstract
Objectives: To describe the design of a clinical trial testing the hypothesis that children randomized to tight glycemic control with intensive insulin therapy after cardiac surgery will have improved clinical outcomes compared to children randomized to conventional blood glucose management.
Design: Two-center, randomized controlled trial.
Setting: Cardiac ICUs at two large academic pediatric centers.
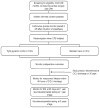
Patients: Children from birth to those aged 36 months recovering in the cardiac ICU after surgery with cardiopulmonary bypass.
Interventions: Subjects in the tight glycemic control (intervention) group receive an intravenous insulin infusion titrated to achieve normoglycemia (target blood glucose range of 80-110 mg/dL; 4.4-6.1 mmol/L). The intervention begins at admission to the cardiac ICU from the operating room and terminates when the patient is ready for discharge from the ICU. Continuous glucose monitoring is performed during insulin infusion to minimize the risks of hypoglycemia. The standard care group has no target blood glucose range.
Measurements and main results: The primary outcome is the development of any nosocomial infection (bloodstream, urinary tract, and surgical site infection or nosocomial pneumonia). Secondary outcomes include mortality, measures of cardiorespiratory function and recovery, laboratory indices of nutritional balance, immunologic, endocrinologic, and neurologic function, cardiac ICU and hospital length of stay, and neurodevelopmental outcome at 1 and 3 yrs of age. A total of 980 subjects will be enrolled (490 in each treatment arm) for sufficient power to show a 50% reduction in the prevalence of the primary outcome.
Conclusions: Pediatric cardiac surgery patients may recognize great benefit from tight glycemic control in the postoperative period, particularly with regard to reduction of nosocomial infections. The Safe Pediatric Euglycemia after Cardiac Surgery trial is designed to provide an unbiased answer to the question of whether this therapy is indeed beneficial and to define the associated risks of therapy.
Conflict of interest statement
Conflicts of interest: Michael S.D. Agus is a paid consultant to two glucose monitoring companies to aid in developing improved glucose measurement techniques in the ICU (Roche Diagnostics, Medtronic Diabetes).
Figures
Comment in
-
Examining the "SPECS" of the safe pediatric euglycemia after cardiac surgery study: thinking outside the box for safer control of blood glucose in the ICU.Pediatr Crit Care Med. 2013 Feb;14(2):226-7. doi: 10.1097/PCC.0b013e3182677468. Pediatr Crit Care Med. 2013. PMID: 23388571 No abstract available.
References
-
- Ballweg JA, Wernovsky G, Ittenbach RF, Bernbaum J, Gerdes M, Gallagher PR, Dominguez TE, Zackai E, Clancy RR, Nicolson SC, et al. Hyperglycemia after infant cardiac surgery does not adversely impact neurodevelopmental outcome. Ann Thorac Surg. 2007;84(6):2052–2058. - PubMed
-
- Moga MA, Manlhiot C, Marwali EM, McCrindle BW, Van Arsdell GS, Schwartz SM. Hyperglycemia after pediatric cardiac surgery: Impact of age and residual lesions. Crit Care Med. 39(2):266–272. - PubMed
-
- Ulate KP, Lima Falcao GC, Bielefeld MR, Morales JM, Rotta AT. Strict glycemic targets need not be so strict: a more permissive glycemic range for critically ill children. Pediatrics. 2008;122(4):e898–904. - PubMed
-
- Yates AR, Dyke PC, 2nd, Taeed R, Hoffman TM, Hayes J, Feltes TF, Cua CL. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med. 2006;7(4):351–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical