Spatio-temporal development of axonopathy in canine intervertebral disc disease as a translational large animal model for nonexperimental spinal cord injury
- PMID: 22805224
- PMCID: PMC8029293
- DOI: 10.1111/j.1750-3639.2012.00617.x
Spatio-temporal development of axonopathy in canine intervertebral disc disease as a translational large animal model for nonexperimental spinal cord injury
Abstract
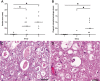
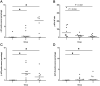
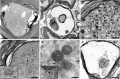
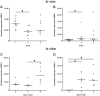
Spinal cord injury (SCI) represents a devastating central nervous system disease that still lacks sufficient therapies. Here, dogs are increasingly recognized as a preclinical animal model for the development of future therapies. The aim of this study was a detailed characterization of axonopathy in canine intervertebral disc disease, which produces a mixed contusive and compressive injury and functions as a spontaneous translational animal model for human SCI. The results revealed an early occurrence of ultrastructurally distinct axonal swelling. Immunohistochemically, enhanced axonal expression of β-amyloid precursor protein, non-phosphorylated neurofilament (n-NF) and growth-associated protein-43 was detected in the epicenter during acute canine SCI. Indicative of a progressive axonopathy, these changes showed a cranial and caudally accentuated spatial progression in the subacute disease phase. In canine spinal cord slice cultures, immunoreactivity of axons was confined to n-NF. Real-time quantitative polymerase chain reaction of naturally traumatized tissue and slice cultures revealed a temporally distinct dysregulation of the matrix metalloproteinases (MMP)-2 and MMP-9 with a dominating expression of the latter. Contrasting to early axonopathy, diminished myelin basic protein immunoreactivity and phagocytosis were delayed. The results present a basis for assessing new therapies in the canine animal model for translational research that might allow partial extrapolation to human SCI.
© 2012 The Authors; Brain Pathology © 2012 International Society of Neuropathology.
Figures


Similar articles
-
Prominent microglial activation in the early proinflammatory immune response in naturally occurring canine spinal cord injury.J Neuropathol Exp Neurol. 2011 Aug;70(8):703-14. doi: 10.1097/NEN.0b013e3182270f8e. J Neuropathol Exp Neurol. 2011. PMID: 21760535
-
Matrix metalloprotease-9 activity in the cerebrospinal fluid and spinal injury severity in dogs with intervertebral disc herniation.Res Vet Sci. 2011 Dec;91(3):482-5. doi: 10.1016/j.rvsc.2010.09.009. Epub 2010 Oct 20. Res Vet Sci. 2011. PMID: 20965533
-
Progressive changes in neurofilament proteins and growth-associated protein-43 immunoreactivities at the site of cervical spinal cord compression in spinal hyperostotic mice.Spine (Phila Pa 1976). 2002 Mar 1;27(5):480-6. doi: 10.1097/00007632-200203010-00008. Spine (Phila Pa 1976). 2002. PMID: 11880833
-
Urological Sequelae to Acute Spinal Cord Injury in Pet Dogs: A Natural Disease Model of Neuropathic Bladder Dysfunction.Top Spinal Cord Inj Rehabil. 2019 Summer;25(3):205-213. doi: 10.1310/sci2503-205. Top Spinal Cord Inj Rehabil. 2019. PMID: 31548787 Free PMC article. Review.
-
Beyond the Laboratory, Into the Clinic: What Dogs with Disk Disease Have Taught Us About Photobiomodulation for Spinal Cord Injury.Photomed Laser Surg. 2017 Nov;35(11):589-594. doi: 10.1089/pho.2017.4348. Photomed Laser Surg. 2017. PMID: 29099681 Review.
Cited by
-
Characterization of microglia/macrophage phenotypes in the spinal cord following intervertebral disc herniation.Front Vet Sci. 2022 Oct 3;9:942967. doi: 10.3389/fvets.2022.942967. eCollection 2022. Front Vet Sci. 2022. PMID: 36262531 Free PMC article.
-
Spontaneous acute and chronic spinal cord injuries in paraplegic dogs: a comparative study of in vivo diffusion tensor imaging.Spinal Cord. 2017 Dec;55(12):1108-1116. doi: 10.1038/sc.2017.83. Epub 2017 Aug 1. Spinal Cord. 2017. PMID: 28762382
-
Arachidonic acid pathway alterations in cerebrospinal fluid of dogs with naturally occurring spinal cord injury.BMC Neurosci. 2016 Jun 10;17(1):31. doi: 10.1186/s12868-016-0269-4. BMC Neurosci. 2016. PMID: 27287721 Free PMC article.
-
Current Insights Into the Pathology of Canine Intervertebral Disc Extrusion-Induced Spinal Cord Injury.Front Vet Sci. 2020 Oct 27;7:595796. doi: 10.3389/fvets.2020.595796. eCollection 2020. Front Vet Sci. 2020. PMID: 33195632 Free PMC article. Review.
-
Cerebrospinal fluid inflammatory cytokines and chemokines in naturally occurring canine spinal cord injury.J Neurotrauma. 2014 Sep 15;31(18):1561-9. doi: 10.1089/neu.2014.3405. Epub 2014 Jul 8. J Neurotrauma. 2014. PMID: 24786364 Free PMC article.
References
-
- Ahlgren S, Li GL, Olsson Y (1996) Accumulation of beta‐amyloid precursor protein and ubiquitin in axons after spinal cord trauma in humans: immunohistochemical observations on autopsy material. Acta Neuropathol 92:49–55. - PubMed
-
- Andrade MS, Mendonça LM, Chadi G (2010) Treadmill running protects spinal cord contusion from secondary degeneration. Brain Res 1346:266–278. - PubMed
-
- Andrews MR, Stelzner DJ (2007) Evaluation of olfactory ensheathing and Schwann cells after implantation into a dorsal injury of adult rat spinal cord. J Neurotrauma 24:1773–1792. - PubMed
-
- Anthes DL, Theriault E, Tator CH (1995) Characterization of axonal ultrastructural pathology following experimental spinal cord compression injury. Brain Res 702:1–16. - PubMed
-
- Bareyre FM, Schwab ME (2003) Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci 26:555–563. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

