Combination of aprepitant, palonosetron and dexamethasone as antiemetic prophylaxis in lung cancer patients receiving multiple cycles of cisplatin-based chemotherapy
- PMID: 22805267
- PMCID: PMC3437500
- DOI: 10.1111/j.1742-1241.2012.02969.x
Combination of aprepitant, palonosetron and dexamethasone as antiemetic prophylaxis in lung cancer patients receiving multiple cycles of cisplatin-based chemotherapy
Abstract
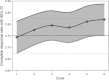
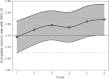
Introduction: With repeated courses of chemotherapy, chemotherapy-induced nausea and vomiting (CINV) becomes progressively more difficult to control. The aim of this study was to evaluate whether the antiemetic efficacy of the triple combination aprepitant, palonosetron and dexamethasone could be sustained for up to six cycles of highly emetogenic chemotherapy (HEC) (cisplatin ≥ 50 mg/m(2) ). Methods: Chemotherapy-naive patients receiving cisplatin-based HEC, were treated with palonosetron 0.25 mg/i.v., dexamethasone 20 mg/i.v. and aprepitant 125 mg/p.o. 1 h before chemotherapy. Aprepitant 80 mg/p.o. and dexamethasone 4 mg/p.o. were administered on days 2-3. The primary endpoint was complete response (CR, no vomiting and no use of rescue medication), over 5 days following HEC in up to six cycles. Secondary endpoints were emesis-free and nausea-free rates. Safety was also evaluated. Results: One hundred and fifty six lung cancer patients were included in the study; the median age was 64 years and 76.9% were men. The minimum cisplatin dosage was 75 mg/m(2) , and in most patients was combined with another drug (87.4%). CR ranged from 74.4% (first cycle) to 82% (sixth cycle). More than 90% and 60% of patients were emesis-free and nausea-free during all chemotherapy cycles. The most commonly reported side effects were constipation and headache. Conclusions: The triple combination of aprepitant, palonosetron and dexamethasone enhanced not only the antiemetic protection during the first cycle, but its efficacy was also sustained for up to six cycles of cisplatin-based HEC in lung cancer patients.
© 2012 Blackwell Publishing Ltd.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer Statistic, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Dong X, Huang J, Cao R, et al. Palonosetron for prevention of acute and delayed nausea and vomiting in non-small-cell lung carcinoma patients. Med Oncol. 2010;28(4):1425–9. - PubMed
-
- Feinberg B, Gilmore J, Haislip S, et al. Impact of initiating antiemetic prophylaxis with palonosetron versus ondansetron on risk of uncontrolled chemotherapy-induced nausea and vomiting in patients with lung cancer receiving multi-day chemotherapy. Sup Care Cancer. 2012;20:615–23. - PubMed
-
- Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–43. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous