The FIB-PPH trial: fibrinogen concentrate as initial treatment for postpartum haemorrhage: study protocol for a randomised controlled trial
- PMID: 22805300
- PMCID: PMC3434105
- DOI: 10.1186/1745-6215-13-110
The FIB-PPH trial: fibrinogen concentrate as initial treatment for postpartum haemorrhage: study protocol for a randomised controlled trial
Abstract
Background: Postpartum haemorrhage (PPH) remains a leading cause of maternal mortality worldwide. In Denmark 2% of parturients receive blood transfusion. During the course of bleeding fibrinogen (coagulation factor I) may be depleted and fall to critically low levels, impairing haemostasis and thus worsening the ongoing bleeding. A plasma level of fibrinogen below 2 g/L in the early phase of postpartum haemorrhage is associated with subsequent development of severe haemorrhage. Use of fibrinogen concentrate allows high-dose substitution without the need for blood type crossmatch. So far no publications of randomised controlled trials involving acutely bleeding patients in the obstetrical setting have been published. This trial aims to investigate if early treatment with fibrinogen concentrate reduces the need for blood transfusion in women suffering severe PPH.
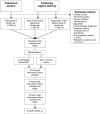
Methods/design: In this randomised placebo-controlled double-blind multicentre trial, parturients with primary PPH are eligible following vaginal delivery in case of: manual removal of placenta (blood loss ≥ 500 ml) or manual exploration of the uterus after the birth of placenta (blood loss ≥ 1000 ml). Caesarean sections are also eligible in case of perioperative blood loss ≥ 1000 ml. The exclusion criteria are known inherited haemostatic deficiencies, prepartum treatment with antithrombotics, pre-pregnancy weight <45 kg or refusal to receive blood transfusion. Following informed consent, patients are randomly allocated to either early treatment with 2 g fibrinogen concentrate or 100 ml isotonic saline (placebo). Haemostatic monitoring with standard laboratory coagulation tests and thromboelastography (TEG, functional fibrinogen and Rapid TEG) is performed during the initial 24 hours.Primary outcome is the need for blood transfusion. To investigate a 33% reduction in the need for blood transfusion, a total of 245 patients will be included. Four university-affiliated public tertiary care hospitals will include patients during a two-year period. Adverse events including thrombosis are assessed in accordance with International Conference on Harmonisation (ICH) good clinical practice (GCP).
Discussion: A widespread belief in the benefits of early fibrinogen substitution in cases of PPH has led to increased off-label use. The FIB-PPH trial is investigator-initiated and aims to provide an evidence-based platform for the recommendations of the early use of fibrinogen concentrate in PPH.
Trial registration: ClincialTrials.gov NCT01359878.
Figures
References
-
- Paidas MJ, Hossain N, Shamsi TS, Rodger MA, Langhoff-Roos J, Lockwood CJ. Hemostasis and Thrombosis in Obstetrics & Gynecology. Wiley-Blackwell, Oxford; 2011.
-
- Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2007. p. CD003249. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

