Regenerative medicine for the treatment of spinal cord injury: more than just promises?
- PMID: 22805417
- PMCID: PMC4118226
- DOI: 10.1111/j.1582-4934.2012.01603.x
Regenerative medicine for the treatment of spinal cord injury: more than just promises?
Abstract
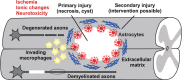
Spinal cord injury triggers a complex set of events that lead to tissue healing without the restoration of normal function due to the poor regenerative capacity of the spinal cord. Nevertheless, current knowledge about the intrinsic regenerative ability of central nervous system axons, when in a supportive environment, has made the prospect of treating spinal cord injury a reality. Among the range of strategies under investigation, cell-based therapies offer the most promising results, due to the multifactorial roles that these cells can fulfil. However, the best cell source is still a matter of debate, as are clinical issues that include the optimal cell dose as well as the timing and route of administration. In this context, the role of biomaterials is gaining importance. These can not only act as vehicles for the administered cells but also, in the case of chronic lesions, can be used to fill the permanent cyst, thus creating a more favourable and conducive environment for axonal regeneration in addition to serving as local delivery systems of therapeutic agents to improve the regenerative milieu. Some of the candidate molecules for the future are discussed in view of the knowledge derived from studying the mechanisms that facilitate the intrinsic regenerative capacity of central nervous system neurons. The future challenge for the multidisciplinary teams working in the field is to translate the knowledge acquired in basic research into effective combinatorial therapies to be applied in the clinic.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



References
-
- Ronaghi M, Erceg S, Moreno-Manzano V, et al. Challenges of stem cell therapy for spinal cord injury: human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells. 2010;28:93–9. - PubMed
-
- Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–9. - PubMed
-
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;26:238–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

