Epigenetics: judge, jury and executioner of stem cell fate
- PMID: 22805743
- PMCID: PMC3427278
- DOI: 10.4161/epi.21141
Epigenetics: judge, jury and executioner of stem cell fate
Abstract
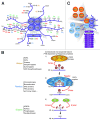
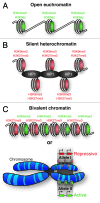
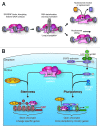
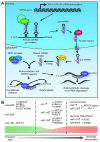
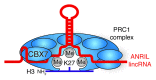
Emerging evidence is shedding light on a large and complex network of epigenetic modifications at play in human stem cells. This "epigenetic landscape" governs the fine-tuning and precision of gene expression programs that define the molecular basis of stem cell pluripotency, differentiation and reprogramming. This review will focus on recent progress in our understanding of the processes that govern this landscape in stem cells, such as histone modification, DNA methylation, alterations of chromatin structure due to chromatin remodeling and non-coding RNA activity. Further investigation into stem cell epigenetics promises to provide novel advances in the diagnosis and treatment of a wide array of human diseases.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
