Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent
- PMID: 22806357
- PMCID: PMC3493679
- DOI: 10.1002/hep.25938
Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent
Abstract
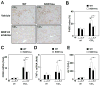
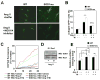
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) generates reactive oxygen species (ROS) in hepatic stellate cells (HSCs) during liver fibrosis. In response to fibrogenic agonists, such as angiotensin II (Ang II), the NOX1 components form an active complex, including Ras-related botulinum toxin substrate 1 (Rac1). Superoxide dismutase 1 (SOD1) interacts with the NOX-Rac1 complex to stimulate NOX activity. NOX4 is also induced in activated HSCs/myofibroblast by increased gene expression. Here, we investigate the role of an enhanced activity SOD1 G37R mutation (SODmu) and the effects of GKT137831, a dual NOX1/4 inhibitor, on HSCs and liver fibrosis. To induce liver fibrosis, wild-type (WT) and SOD1mu mice were treated with CCl(4) or bile duct ligation (BDL). Then, to address the role of NOX-SOD1-mediated ROS production in HSC activation and liver fibrosis, mice were treated with a NOX1/4 inhibitor. Fibrosis and ROS generation was assessed by histology and measurement of thiobarbituric acid reactive substances and NOX-related genes. Primary cultured HSCs isolated from WT, SODmu, and NOX1 knockout (KO) mice were assessed for ROS production, Rac1 activity, and NOX gene expression. Liver fibrosis was increased in SOD1mu mice, and ROS production and Rac1 activity were increased in SOD1mu HSCs. The NOX1/4 inhibitor, GKT137831, attenuated liver fibrosis and ROS production in both SOD1mu and WT mice as well as messenger RNA expression of fibrotic and NOX genes. Treatment with GKT137831 suppressed ROS production and NOX and fibrotic gene expression, but not Rac1 activity, in SOD1mut and WT HSCs. Both Ang II and tumor growth factor beta up-regulated NOX4, but Ang II required NOX1.
Conclusions: SOD1mu induces excessive NOX1 activation through Rac1 in HSCs, causing enhanced NOX4 up-regulation, ROS generation, and liver fibrosis. Treatment targeting NOX1/4 may be a new therapy for liver fibrosis.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures





References
-
- Fink SA, Jacobson IM. Managing patients with hepatitisB-related or hepatitisC-related decompensated cirrhosis. Nat Rev Gastroenterol Hepatol. 2011;8:285–295. - PubMed
-
- Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, Seki E, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–1572. - PubMed
-
- Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
