Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8⁺ T-cell responses in melanoma patients
- PMID: 22806397
- PMCID: PMC3549564
- DOI: 10.1002/eji.201142361
Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8⁺ T-cell responses in melanoma patients
Erratum in
-
Corrections.Eur J Immunol. 2016 Feb;46(2):493. doi: 10.1002/eji.201670027. Eur J Immunol. 2016. PMID: 26840199 Free PMC article. No abstract available.
-
Corrections.Eur J Immunol. 2016 Feb;46(2):493. doi: 10.1002/eji.201670026. Eur J Immunol. 2016. PMID: 26840200 Free PMC article. No abstract available.
Abstract
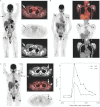
Optimal vaccine strategies must be identified for improving T-cell vaccination against infectious and malignant diseases. MelQbG10 is a virus-like nano-particle loaded with A-type CpG-oligonucleotides (CpG-ODN) and coupled to peptide(16-35) derived from Melan-A/MART-1. In this phase IIa clinical study, four groups of stage III-IV melanoma patients were vaccinated with MelQbG10, given (i) with IFA (Montanide) s.c.; (ii) with IFA s.c. and topical Imiquimod; (iii) i.d. with topical Imiquimod; or (iv) as intralymph node injection. In total, 16/21 (76%) patients generated ex vivo detectable Melan-A/MART-1-specific T-cell responses. T-cell frequencies were significantly higher when IFA was used as adjuvant, resulting in detectable T-cell responses in all (11/11) patients, with predominant generation of effector-memory-phenotype cells. In turn, Imiquimod induced higher proportions of central-memory-phenotype cells and increased percentages of CD127(+) (IL-7R) T cells. Direct injection of MelQbG10 into lymph nodes resulted in lower T-cell frequencies, associated with lower proportions of memory and effector-phenotype T cells. Swelling of vaccine site draining lymph nodes, and increased glucose uptake at PET/CT was observed in 13/15 (87%) of evaluable patients, reflecting vaccine triggered immune reactions in lymph nodes. We conclude that the simultaneous use of both Imiquimod and CpG-ODN induced combined memory and effector CD8(+) T-cell responses.
© 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
-
- Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, Lebbe C, Baurain JF, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–26. - PubMed
-
- Eggermont AM. Immunotherapy: vaccine trials in melanoma – time for reflection. Nat. Rev. Clin. Oncol. 2009;6:256–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

