Architecture and regulation of HtrA-family proteins involved in protein quality control and stress response
- PMID: 22806565
- PMCID: PMC11113883
- DOI: 10.1007/s00018-012-1076-4
Architecture and regulation of HtrA-family proteins involved in protein quality control and stress response
Abstract
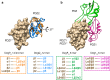
Protein quality control is vital for all living cells and sophisticated molecular mechanisms have evolved to prevent the excessive accumulation of unfolded proteins. High-temperature requirement A (HtrA) proteases have been identified as important ATP-independent quality-control factors in most species. HtrA proteins harbor a serine-protease domain and at least one peptide-binding PDZ domain to ensure efficient removal of misfolded or damaged proteins. One distinctive property of HtrAs is their ability to assemble into complex oligomers. Whereas all examined HtrAs are capable of forming pyramidal 3-mers, higher-order complexes consisting of up to 24 molecules have been reported. Tight control of chaperone and protease function is of pivotal importance in preventing deleterious HtrA-protease activity. In recent years, structural biology provided detailed insights into the molecular basis of the regulatory mechanisms, which include unique intramolecular allosteric signaling cascades and the dynamic switching of oligomeric states of HtrA proteins. Based on these results, functional models for many family members have been developed. The HtrA protein family represents a remarkable example of how structural and functional diversity is attained from the assembly of simple molecular building blocks.
Figures




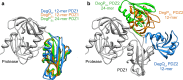
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

