Role of SHP2 phosphatase in KIT-induced transformation: identification of SHP2 as a druggable target in diseases involving oncogenic KIT
- PMID: 22806893
- PMCID: PMC3460688
- DOI: 10.1182/blood-2011-08-375873
Role of SHP2 phosphatase in KIT-induced transformation: identification of SHP2 as a druggable target in diseases involving oncogenic KIT
Abstract
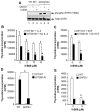
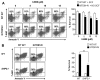
Intracellular mechanism(s) that contribute to promiscuous signaling via oncogenic KIT in systemic mastocytosis and acute myelogenous leukemia are poorly understood. We show that SHP2 phosphatase is essential for oncogenic KIT-induced growth and survival in vitro and myeloproliferative disease (MPD) in vivo. Genetic disruption of SHP2 or treatment of oncogene-bearing cells with a novel SHP2 inhibitor alone or in combination with the PI3K inhibitor corrects MPD by disrupting a protein complex involving p85α, SHP2, and Gab2. Importantly, a single tyrosine at position 719 in oncogenic KIT is sufficient to develop MPD by recruiting p85α, SHP2, and Gab2 complex to oncogenic KIT. Our results demonstrate that SHP2 phosphatase is a druggable target that cooperates with lipid kinases in inducing MPD.
Figures





Comment in
-
KIT's ship comes in.Blood. 2012 Sep 27;120(13):2541-2. doi: 10.1182/blood-2012-08-445247. Blood. 2012. PMID: 23019203 No abstract available.
References
-
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. - PubMed
-
- Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001;25(7):571–576. - PubMed
-
- Beghini A, Peterlongo P, Ripamonti CB, et al. C-kit mutations in core binding factor leukemias. Blood. 2000;95(2):726–727. - PubMed
-
- Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92(23):10560–10564. - PMC - PubMed
-
- Kitayama H, Kanakura Y, Furitsu T, et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood. 1995;85(3):790–798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

