Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1-, IL-17-, and G-CSF-dependent manner
- PMID: 22806894
- PMCID: PMC3460682
- DOI: 10.1182/blood-2012-02-409805
Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1-, IL-17-, and G-CSF-dependent manner
Abstract
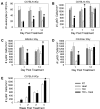
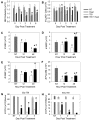
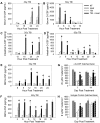
Neutropenia is a common side effect of cytotoxic chemotherapy and radiation, increasing the risk of infection in these patients. Here we examined the impact of body temperature on neutrophil recovery in the blood and bone marrow after total body irradiation (TBI). Mice were exposed to either 3 or 6 Gy TBI followed by a mild heat treatment that temporarily raised core body temperature to approximately 39.5°C. Neutrophil recovery was then compared with control mice that received either TBI alone heat treatment alone. Mice that received both TBI and heat treatment exhibited a significant increase in the rate of neutrophil recovery in the blood and an increase in the number of marrow hematopoietic stem cells and neutrophil progenitors compared with that seen in mice that received either TBI or heat alone. The combination treatment also increased G-CSF concentrations in the serum, bone marrow, and intestinal tissue and IL-17, IL-1β, and IL-1α concentrations in the intestinal tissue after TBI. Neutralizing G-CSF or inhibiting IL-17 or IL-1 signaling significantly blocked the thermally mediated increase in neutrophil numbers. These findings suggest that a physiologically relevant increase in body temperature can accelerate recovery from neutropenia after TBI through a G-CSF-, IL-17-, and IL-1-dependent mechanism.
Figures



Comment in
-
Keep up the heat on IL-1.Blood. 2012 Sep 27;120(13):2538-9. doi: 10.1182/blood-2012-08-445254. Blood. 2012. PMID: 23019200 No abstract available.
References
-
- Kaplan HS, Speck RS, Jawetz E. Impairment of antimicrobial defense following total body irradiation of mice. J Lab Clin Med. 1952;40(5):682–691. - PubMed
-
- Brook I. Use of antibiotics in the management of postirradiation wound infection and sepsis. Radiat Res. 1988;115(1):1–25. - PubMed
-
- Segal BH, Freifeld AG, Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2008;6(2):122–174. - PubMed
-
- Dale DC. Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs. 2002;62(Suppl 1):1–15. - PubMed
-
- Richards MK, Liu F, Iwasaki H, Akashi K, Link DC. Pivotal role of granulocyte colony-stimulating factor in the development of progenitors in the common myeloid pathway. Blood. 2003;102(10):3562–3568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

