In vivo-formed versus preformed metabolite kinetics of trans-resveratrol-3-sulfate and trans-resveratrol-3-glucuronide
- PMID: 22807110
- PMCID: PMC3463825
- DOI: 10.1124/dmd.112.046417
In vivo-formed versus preformed metabolite kinetics of trans-resveratrol-3-sulfate and trans-resveratrol-3-glucuronide
Erratum in
- Drug Metab Dispos. 2012 Dec;40(12):2382
Abstract
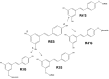
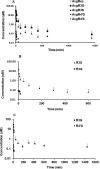
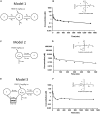
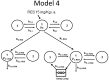
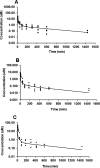
Metabolites in safety testing have gained a lot of attention recently. Regulatory agencies have suggested that the kinetics of preformed and in vivo-formed metabolites are comparable. This subject has been a topic of debate. We have compared the kinetics of in vivo-formed with preformed metabolites. trans-3,5,4'-Trihydroxystilbene [trans-resveratrol (RES)] and its two major metabolites, resveratrol-3-sulfate (R3S) and resveratrol-3-glucuronide (R3G) were used as model substrates. The pharmacokinetics (PK) of R3S and R3G were characterized under two situations. First, the pharmacokinetics of R3S and R3G were characterized (in vivo-formed metabolite) after administration of RES. Then, synthetic R3S and R3G were administered (preformed metabolite) and their pharmacokinetics were characterized. PK models were developed to describe the data. A three-compartment model for RES, a two-compartment model for R3S (preformed), and an enterohepatic cycling model for R3G (preformed) was found to describe the data well. These three models were further combined to build a comprehensive PK model, which was used to perform simulations to predict in vivo-formed metabolite kinetics. Comparisons were made between in vivo-formed and preformed metabolite kinetics. Marked differences were observed in the kinetics of preformed and in vivo-formed metabolites.
Figures

Similar articles
-
Pulmonary metabolism of resveratrol: in vitro and in vivo evidence.Drug Metab Dispos. 2013 May;41(5):1163-9. doi: 10.1124/dmd.113.051326. Epub 2013 Mar 8. Drug Metab Dispos. 2013. PMID: 23474649 Free PMC article.
-
Analytical method development for synthesized conjugated metabolites of trans-resveratrol, and application to pharmacokinetic studies.J Pharm Biomed Anal. 2012 Apr 7;63:1-8. doi: 10.1016/j.jpba.2011.12.006. Epub 2011 Dec 16. J Pharm Biomed Anal. 2012. PMID: 22342060 Free PMC article.
-
Population pharmacokinetic modeling of trans-resveratrol and its glucuronide and sulfate conjugates after oral and intravenous administration in rats.Pharm Res. 2011 Jul;28(7):1606-21. doi: 10.1007/s11095-011-0395-8. Epub 2011 Mar 23. Pharm Res. 2011. PMID: 21431452
-
Safety testing of metabolites: Expectations and outcomes.Chem Biol Interact. 2009 Apr 15;179(1):45-59. doi: 10.1016/j.cbi.2008.09.013. Epub 2008 Sep 23. Chem Biol Interact. 2009. PMID: 18926805 Review.
-
Metabolism and pharmacokinetics of resveratrol and pterostilbene.Biofactors. 2018 Jan;44(1):16-25. doi: 10.1002/biof.1410. Epub 2018 Jan 8. Biofactors. 2018. PMID: 29315886 Review.
Cited by
-
Differential sensitivities of bladder cancer cell lines to resveratol are unrelated to its metabolic profile.Oncotarget. 2017 Jun 20;8(25):40289-40304. doi: 10.18632/oncotarget.15041. Oncotarget. 2017. PMID: 28178690 Free PMC article.
-
Chemopreventive efficacy of oral curcumin: a prodrug hypothesis.FASEB J. 2019 Aug;33(8):9453-9465. doi: 10.1096/fj.201900166R. Epub 2019 May 28. FASEB J. 2019. PMID: 31136203 Free PMC article.
-
New water-soluble carbamate ester derivatives of resveratrol.Molecules. 2014 Oct 1;19(10):15900-17. doi: 10.3390/molecules191015900. Molecules. 2014. PMID: 25275336 Free PMC article.
-
Pulmonary metabolism of resveratrol: in vitro and in vivo evidence.Drug Metab Dispos. 2013 May;41(5):1163-9. doi: 10.1124/dmd.113.051326. Epub 2013 Mar 8. Drug Metab Dispos. 2013. PMID: 23474649 Free PMC article.
-
"Target-Site" Drug Metabolism and Transport.Drug Metab Dispos. 2015 Aug;43(8):1156-68. doi: 10.1124/dmd.115.064576. Epub 2015 May 18. Drug Metab Dispos. 2015. PMID: 25986849 Free PMC article.
References
-
- Akaike H. (1974) A new look at the statistical model identification. IEEE Trans Automat Contr AC-19:716–723
-
- Baillie TA, Cayen MN, Fouda H, Gerson RJ, Green JD, Grossman SJ, Klunk LJ, LeBlanc B, Perkins DG, Shipley LA. (2002) Drug metabolites in safety testing. Toxicol Appl Pharmacol 182:188–196 - PubMed
-
- Berezhkovskiy LM. (2004) Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. J Pharm Sci 93:1628–1640 - PubMed
-
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, et al. (2007) Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 16:1246–1252 - PubMed
-
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, et al. (2010) Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res 70:9003–9011 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

