RPGR-associated retinal degeneration in human X-linked RP and a murine model
- PMID: 22807293
- PMCID: PMC3422104
- DOI: 10.1167/iovs.12-10070
RPGR-associated retinal degeneration in human X-linked RP and a murine model
Abstract
Purpose: We investigated the retinal disease due to mutations in the retinitis pigmentosa GTPase regulator (RPGR) gene in human patients and in an Rpgr conditional knockout (cko) mouse model.
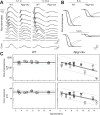
Methods: XLRP patients with RPGR-ORF15 mutations (n = 35, ages at first visit 5-72 years) had clinical examinations, and rod and cone perimetry. Rpgr-cko mice, in which the proximal promoter and first exon were deleted ubiquitously, were back-crossed onto a BALB/c background, and studied with optical coherence tomography and electroretinography (ERG). Retinal histopathology was performed on a subset.
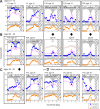
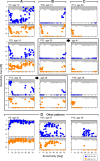
Results: Different patterns of rod and cone dysfunction were present in patients. Frequently, there were midperipheral losses with residual rod and cone function in central and peripheral retina. Longitudinal data indicated that central rod loss preceded peripheral rod losses. Central cone-only vision with no peripheral function was a late stage. Less commonly, patients had central rod and cone dysfunction, but preserved, albeit abnormal, midperipheral rod and cone vision. Rpgr-cko mice had progressive retinal degeneration detectable in the first months of life. ERGs indicated relatively equal rod and cone disease. At late stages, there was greater inferior versus superior retinal degeneration.
Conclusions: RPGR mutations lead to progressive loss of rod and cone vision, but show different patterns of residual photoreceptor disease expression. Knowledge of the patterns should guide treatment strategies. Rpgr-cko mice had onset of degeneration at relatively young ages and progressive photoreceptor disease. The natural history in this model will permit preclinical proof-of-concept studies to be designed and such studies should advance progress toward human therapy.
Conflict of interest statement
Disclosure:
Figures



References
-
- Wright AF. Towards the identification of genes in X-linked retinitis pigmentosa. Prog Ret Res. 1990;9:197–227
-
- Wright AF, Shu X. Focus on molecules: RPGR. Exp Eye Res. 2007;85:1–2 - PubMed
-
- Shu X, Black GC, Rice JM, et al. RPGR mutation analysis and disease: an update. Hum Mutat. 2007;28:322–328 - PubMed
-
- Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007;48:1298–1304 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

