Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter
- PMID: 22807325
- PMCID: PMC3405197
- DOI: 10.1002/ana.23651
Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter
Abstract
Objective: Aquaporin 4 (AQP4)-specific autoantibodies in neuromyelitis optica (NMO) are immunoglobulin (Ig)G1, a T cell-dependent Ig subclass, indicating that AQP4-specific T cells participate in NMO pathogenesis. Our goal was to identify and characterize AQP4-specific T cells in NMO patients and healthy controls (HC).
Methods: Peripheral blood T cells from NMO patients and HC were examined for recognition of AQP4 and production of proinflammatory cytokines. Monocytes were evaluated for production of T cell-polarizing cytokines and expression of costimulatory molecules.
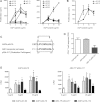
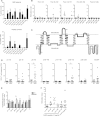
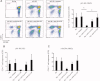
Results: T cells from NMO patients and HC proliferated to intact AQP4 or AQP4 peptides (p11-30, p21-40, p61-80, p131-150, p156-170, p211-230, and p261-280). T cells from NMO patients demonstrated greater proliferation to AQP4 than those from HC, and responded most vigorously to p61-80, a naturally processed immunodominant determinant of intact AQP4. T cells were CD4(+), and corresponding to association of NMO with human leukocyte antigen (HLA)-DRB1*0301 and DRB3, AQP4 p61-80-specific T cells were HLA-DR restricted. The T-cell epitope within AQP4 p61-80 was mapped to 63-76, which contains 10 residues with 90% homology to a sequence within Clostridium perfringens adenosine triphosphate-binding cassette (ABC) transporter permease. T cells from NMO patients proliferated to this homologous bacterial sequence, and cross-reactivity between it and self-AQP4 was observed, supporting molecular mimicry. In NMO, AQP4 p61-80-specific T cells exhibited Th17 polarization, and furthermore, monocytes produced more interleukin 6, a Th17-polarizing cytokine, and expressed elevated CD40 and CD80 costimulatory molecules, suggesting innate immunologic dysfunction.
Interpretation: AQP4-specific T-cell responses are amplified in NMO, exhibit a Th17 bias, and display cross-reactivity to a protein of an indigenous intestinal bacterium, providing new perspectives for investigating NMO pathogenesis.
Copyright © 2012 American Neurological Association.
Figures


References
-
- Bradl M, Misu T, Takahashi T, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

