Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1
- PMID: 22807677
- PMCID: PMC3395602
- DOI: 10.1371/journal.ppat.1002795
Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1
Abstract
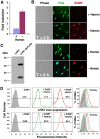
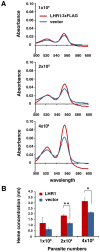
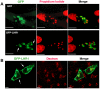
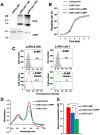
Trypanosomatid protozoan parasites lack a functional heme biosynthetic pathway, so must acquire heme from the environment to survive. However, the molecular pathway responsible for heme acquisition by these organisms is unknown. Here we show that L. amazonensis LHR1, a homolog of the C. elegans plasma membrane heme transporter HRG-4, functions in heme transport. Tagged LHR1 localized to the plasma membrane and to endocytic compartments, in both L. amazonensis and mammalian cells. Heme deprivation in L. amazonensis increased LHR1 transcript levels, promoted uptake of the fluorescent heme analog ZnMP, and increased the total intracellular heme content of promastigotes. Conversely, deletion of one LHR1 allele reduced ZnMP uptake and the intracellular heme pool by approximately 50%, indicating that LHR1 is a major heme importer in L. amazonensis. Viable parasites with correct replacement of both LHR1 alleles could not be obtained despite extensive attempts, suggesting that this gene is essential for the survival of promastigotes. Notably, LHR1 expression allowed Saccharomyces cerevisiae to import heme from the environment, and rescued growth of a strain deficient in heme biosynthesis. Syntenic genes with high sequence identity to LHR1 are present in the genomes of several species of Leishmania and also Trypanosoma cruzi and Trypanosoma brucei, indicating that therapeutic agents targeting this transporter could be effective against a broad group of trypanosomatid parasites that cause serious human disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The Heme Transport Capacity of LHR1 Determines the Extent of Virulence in Leishmania amazonensis.PLoS Negl Trop Dis. 2015 May 22;9(5):e0003804. doi: 10.1371/journal.pntd.0003804. eCollection 2015 May. PLoS Negl Trop Dis. 2015. PMID: 26001191 Free PMC article.
-
Heme uptake mediated by LHR1 is essential for Leishmania amazonensis virulence.Infect Immun. 2013 Oct;81(10):3620-6. doi: 10.1128/IAI.00687-13. Epub 2013 Jul 22. Infect Immun. 2013. PMID: 23876801 Free PMC article.
-
Leishmania heme uptake involves LmFLVCRb, a novel porphyrin transporter essential for the parasite.Cell Mol Life Sci. 2020 May;77(9):1827-1845. doi: 10.1007/s00018-019-03258-3. Epub 2019 Aug 1. Cell Mol Life Sci. 2020. PMID: 31372684 Free PMC article.
-
Pathways of iron acquisition and utilization in Leishmania.Curr Opin Microbiol. 2013 Dec;16(6):716-21. doi: 10.1016/j.mib.2013.07.018. Epub 2013 Aug 17. Curr Opin Microbiol. 2013. PMID: 23962817 Free PMC article. Review.
-
Protean permeases: Diverse roles for membrane transport proteins in kinetoplastid protozoa.Mol Biochem Parasitol. 2019 Jan;227:39-46. doi: 10.1016/j.molbiopara.2018.12.006. Epub 2018 Dec 24. Mol Biochem Parasitol. 2019. PMID: 30590069 Free PMC article. Review.
Cited by
-
Transport proteins of parasitic protists and their role in nutrient salvage.Front Plant Sci. 2014 Apr 29;5:153. doi: 10.3389/fpls.2014.00153. eCollection 2014. Front Plant Sci. 2014. PMID: 24808897 Free PMC article. Review.
-
Understanding How Minerals Contribute to Optimal Immune Function.J Immunol Res. 2023 Nov 1;2023:3355733. doi: 10.1155/2023/3355733. eCollection 2023. J Immunol Res. 2023. PMID: 37946846 Free PMC article. Review.
-
IRONy OF FATE: role of iron-mediated ROS in Leishmania differentiation.Trends Parasitol. 2013 Oct;29(10):489-96. doi: 10.1016/j.pt.2013.07.007. Epub 2013 Aug 12. Trends Parasitol. 2013. PMID: 23948431 Free PMC article. Review.
-
Make it, take it, or leave it: heme metabolism of parasites.PLoS Pathog. 2013 Jan;9(1):e1003088. doi: 10.1371/journal.ppat.1003088. Epub 2013 Jan 17. PLoS Pathog. 2013. PMID: 23349629 Free PMC article. No abstract available.
-
A new model for Trypanosoma cruzi heme homeostasis depends on modulation of TcHTE protein expression.J Biol Chem. 2020 Sep 18;295(38):13202-13212. doi: 10.1074/jbc.RA120.014574. Epub 2020 Jul 23. J Biol Chem. 2020. PMID: 32709751 Free PMC article.
References
-
- Markle WH, Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69:1455–1460. - PubMed
-
- Sundar S, More DK, Singh MK, Singh VP, Sharma S, et al. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–1107. - PubMed
-
- Jeronimo SM, Duggal P, Braz RF, Cheng C, Monteiro GR, et al. An emerging peri-urban pattern of infection with Leishmania chagasi, the protozoan causing visceral leishmaniasis in northeast Brazil. Scand J Infect Dis. 2004;36:443–449. - PubMed
-
- Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

