4-(2-Benzoyl-benzoyl)-N,N-diphenyl-aniline
- PMID: 22807861
- PMCID: PMC3393304
- DOI: 10.1107/S160053681202466X
4-(2-Benzoyl-benzoyl)-N,N-diphenyl-aniline
Abstract
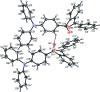
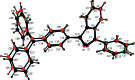
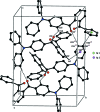
The asymmetric unit of the title compound, C(32)H(23)NO(2), comprises two crystallographically independent mol-ecules. In both mol-ecules, the geometries about the N atoms deviate significantly from the ideal trigonal-planar geometry with bond-angle sums about the N atom of 359.32° in one mol-ecule and 359.86° in the other. The O atoms of the carbonyl groups are deviated significantly from the central benzene rings by 0.6747 (14) and -1.1223 (13) Å in one molecule and -0.6230 (13) and 1.1559 (12) Å in the other. In the diphenyl-aniline units, the terminal phenyl rings are almost orthogonal to each other, with dihedral angles of 89.79 (9) and 89.76 (9)°. The crystal structure features C-H⋯O and C-H⋯π inter-actions.
Figures
References
-
- Bruker (2008). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
-
- Kakimoto, M., Ge, Z. Y., Hayakawa, T., Ando, S. & Ueda, M. (2008). Adv. Funct. Mater. 18, 584–590.
-
- Kennedy, A. R., Smith, W. E., Tackley, D. R., David, W. I. F., Shankland, K., Brown, D. & Teat, S. J. (2002). J. Mater. Chem. 12, 168–172.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
