Crowd sourcing a new paradigm for interactome driven drug target identification in Mycobacterium tuberculosis
- PMID: 22808064
- PMCID: PMC3395720
- DOI: 10.1371/journal.pone.0039808
Crowd sourcing a new paradigm for interactome driven drug target identification in Mycobacterium tuberculosis
Abstract
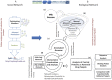
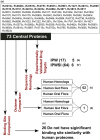
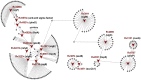
A decade since the availability of Mycobacterium tuberculosis (Mtb) genome sequence, no promising drug has seen the light of the day. This not only indicates the challenges in discovering new drugs but also suggests a gap in our current understanding of Mtb biology. We attempt to bridge this gap by carrying out extensive re-annotation and constructing a systems level protein interaction map of Mtb with an objective of finding novel drug target candidates. Towards this, we synergized crowd sourcing and social networking methods through an initiative 'Connect to Decode' (C2D) to generate the first and largest manually curated interactome of Mtb termed 'interactome pathway' (IPW), encompassing a total of 1434 proteins connected through 2575 functional relationships. Interactions leading to gene regulation, signal transduction, metabolism, structural complex formation have been catalogued. In the process, we have functionally annotated 87% of the Mtb genome in context of gene products. We further combine IPW with STRING based network to report central proteins, which may be assessed as potential drug targets for development of drugs with least possible side effects. The fact that five of the 17 predicted drug targets are already experimentally validated either genetically or biochemically lends credence to our unique approach.
Conflict of interest statement
Figures


References
-
- World Health Organization (WHO) Global tuberculosis control. 2010.
-
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. - PubMed
-
- Camus J-C, Pryor MJ, Médigue C, Cole ST. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002;148:2967–2973. - PubMed
-
- Lew JM, Kapopoulou A, Jones LM, Cole ST. TubercuList –10 years after. Tuberculosis. 2011;91:1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

