A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity
- PMID: 22808149
- PMCID: PMC3396660
- DOI: 10.1371/journal.pone.0040385
A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity
Abstract
Recombinant adenoviruses are among the most promising tools for vaccine antigen delivery. Recently, the development of new vectors has focused on serotypes to which the human population is less exposed in order to circumvent pre-existing anti vector immunity. This study describes the derivation of a new vaccine vector based on a chimpanzee adenovirus, Y25, together with a comparative assessment of its potential to elicit transgene product specific immune responses in mice. The vector was constructed in a bacterial artificial chromosome to facilitate genetic manipulation of genomic clones. In order to conduct a fair head-to-head immunological comparison of multiple adenoviral vectors, we optimised a method for accurate determination of infectious titre, since this parameter exhibits profound natural variability and can confound immunogenicity studies when doses are based on viral particle estimation. Cellular immunogenicity of recombinant E1 E3-deleted vector ChAdY25 was comparable to that of other species E derived chimpanzee adenovirus vectors including ChAd63, the first simian adenovirus vector to enter clinical trials in humans. Furthermore, the prevalence of virus neutralizing antibodies (titre >1:200) against ChAdY25 in serum samples collected from two human populations in the UK and Gambia was particularly low compared to published data for other chimpanzee adenoviruses. These findings support the continued development of new chimpanzee adenovirus vectors, including ChAdY25, for clinical use.
Conflict of interest statement
Figures


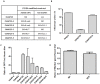
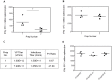
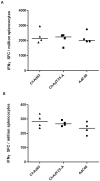
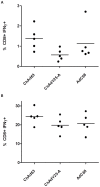

References
-
- Wilson JM. Adenoviruses as gene-delivery vehicles. The New England journal of medicine. 1996;334:1185–1187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

