Impact of combined mTOR and MEK inhibition in uveal melanoma is driven by tumor genotype
- PMID: 22808163
- PMCID: PMC3393714
- DOI: 10.1371/journal.pone.0040439
Impact of combined mTOR and MEK inhibition in uveal melanoma is driven by tumor genotype
Abstract
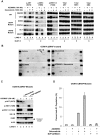
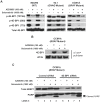
Uveal melanomas possess activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT/mammalian Target of Rapamycin (mTOR) pathways. MAPK activation occurs via somatic mutations in the heterotrimeric G protein subunits GNAQ and GNA11 for over 70% of tumors and less frequently via V600E BRAF mutations. In this report, we describe the impact of dual pathway inhibition upon uveal melanoma cell lines with the MEK inhibitor selumetinib (AZD6244/ARRY-142886) and the ATP-competitive mTOR kinase inhibitor AZD8055. While synergistic reductions in cell viability were observed with AZD8055/selumetinib in both BRAF and GNAQ mutant cell lines, apoptosis was preferentially induced in BRAF mutant cells only. In vitro apoptosis assay results were predictive of in vivo drug efficacy as tumor regressions were observed only in a BRAF mutant xenograft model, but not GNAQ mutant model. We went on to discover that GNAQ promotes relative resistance to AZD8055/selumetinib-induced apoptosis in GNAQ mutant cells. For BRAF mutant cells, both AKT and 4E-BP1 phosphorylation were modulated by the combination; however, decreasing AKT phosphorylation alone was not sufficient and decreasing 4E-BP1 phosphorylation was not required for apoptosis. Instead, cooperative mTOR complex 2 (mTORC2) and MEK inhibition resulting in downregulation of the pro-survival protein MCL-1 was found to be critical for combination-induced apoptosis. These results suggest that the clinical efficacy of combined MEK and mTOR kinase inhibition will be determined by tumor genotype, and that BRAF mutant malignancies will be particularly susceptible to this strategy.
Conflict of interest statement
Figures






References
-
- Patel M, Smyth EC, Chapman PB, Wolchok JD, Schwartz GK, et al. Therapeutic Implications of the Emerging Molecular Biology of Uveal Melanoma. Clin Cancer Res. 2011. - PubMed
-
- Weber A, Hengge UR, Urbanik D, Markwart A, Mirmohammadsaegh A, et al. Absence of mutations of the BRAF gene and constitutive activation of extracellular-regulated kinase in malignant melanomas of the uvea. Lab Invest. 2003;83:1771–1776. - PubMed
-
- Malaponte G, Libra M, Gangemi P, Bevelacqua V, Mangano K, et al. Detection of BRAF gene mutation in primary choroidal melanoma tissue. Cancer Biol Ther. 2006;5:225–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

