Rapid nitration of adipocyte phosphoenolpyruvate carboxykinase by leptin reduces glyceroneogenesis and induces fatty acid release
- PMID: 22808220
- PMCID: PMC3394747
- DOI: 10.1371/journal.pone.0040650
Rapid nitration of adipocyte phosphoenolpyruvate carboxykinase by leptin reduces glyceroneogenesis and induces fatty acid release
Abstract
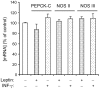
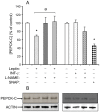
Fatty acid (FA) release from white adipose tissue (WAT) is the result of the balance between triglyceride breakdown and FA re-esterification. The latter relies on the induction of cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C), the key enzyme for glyceroneogenesis. We previously demonstrated that long-term (18 h) leptin treatment of rat epididymal WAT explants reduced glyceroneogenesis through nitric oxide (NO)-induced decrease in PEPCK-C expression. We investigated the effect of a short-term leptin treatment (2 h) on PEPCK-C expression and glyceroneogenesis in relation to NO production. We demonstrate that in WAT explants, leptin-induced NO synthase III (NOS III) phosphorylation was associated with reduced PEPCK-C level and glyceroneogenesis, leading to FA release, while PEPCK-C gene expression remained unaffected. These effects were absent in WAT explants from leptin receptor-deficient Zucker rat. Immunoprecipitation and western blot experiments showed that the leptin-induced decrease in PEPCK-C level was correlated with an increase in PEPCK-C nitration. All these effects were abolished by the NOS inhibitor Nω-nitro-L-arginine methyl ester and mimicked by the NO donor S-nitroso-N-acetyl-DL penicillamine. We propose a mechanism in which leptin activates NOS III and induces NO that nitrates PEPCK-C to reduce its level and glyceroneogenesis, therefore limiting FA re-esterification in WAT.
Conflict of interest statement
Figures



Similar articles
-
Leptin induces nitric oxide-mediated inhibition of lipolysis and glyceroneogenesis in rat white adipose tissue.J Nutr. 2011 Jan;141(1):4-9. doi: 10.3945/jn.110.125765. Epub 2010 Nov 10. J Nutr. 2011. PMID: 21068181
-
Acute and selective inhibition of adipocyte glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase by interferon gamma.Endocrinology. 2007 Aug;148(8):4007-14. doi: 10.1210/en.2006-1760. Epub 2007 May 10. Endocrinology. 2007. PMID: 17495004
-
Acute and selective regulation of glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase in adipose tissue by thiazolidinediones in type 2 diabetes.Diabetologia. 2007 Mar;50(3):666-75. doi: 10.1007/s00125-006-0560-5. Epub 2007 Jan 23. Diabetologia. 2007. PMID: 17242918
-
Fatty acid recycling in adipocytes: a role for glyceroneogenesis and phosphoenolpyruvate carboxykinase.Biochem Soc Trans. 2003 Dec;31(Pt 6):1125-9. doi: 10.1042/bst0311125. Biochem Soc Trans. 2003. PMID: 14641009 Review.
-
Effect of glucocorticoids on glyceroneogenesis in adipose tissue: A systematic review.Biochimie. 2020 Jan;168:210-219. doi: 10.1016/j.biochi.2019.11.007. Epub 2019 Nov 21. Biochimie. 2020. PMID: 31759936
Cited by
-
Leptin as a key regulator of the adipose organ.Rev Endocr Metab Disord. 2022 Feb;23(1):13-30. doi: 10.1007/s11154-021-09687-5. Epub 2021 Sep 14. Rev Endocr Metab Disord. 2022. PMID: 34523036 Free PMC article. Review.
-
Exploration of Genes Related to Intramuscular Fat Deposition in Xinjiang Brown Cattle.Genes (Basel). 2024 Aug 25;15(9):1121. doi: 10.3390/genes15091121. Genes (Basel). 2024. PMID: 39336712 Free PMC article.
-
There and Back Again: Leptin Actions in White Adipose Tissue.Int J Mol Sci. 2020 Aug 21;21(17):6039. doi: 10.3390/ijms21176039. Int J Mol Sci. 2020. PMID: 32839413 Free PMC article. Review.
-
Targeting the NO/superoxide ratio in adipose tissue: relevance to obesity and diabetes management.Br J Pharmacol. 2017 Jun;174(12):1570-1590. doi: 10.1111/bph.13498. Epub 2016 May 15. Br J Pharmacol. 2017. PMID: 27079449 Free PMC article. Review.
-
Oxidative, Reductive, and Nitrosative Stress Effects on Epigenetics and on Posttranslational Modification of Enzymes in Cardiometabolic Diseases.Oxid Med Cell Longev. 2020 Oct 30;2020:8819719. doi: 10.1155/2020/8819719. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33204398 Free PMC article. Review.
References
-
- Boden G. Free fatty acids, insulin resistance, and type 2 diabetes mellitus. Proc Assoc Am Physicians. 1999;111:241–248. - PubMed
-
- McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. - PubMed
-
- Belfrage P, Fredrikson G, Olsson H, Stralfors P. Molecular mechanisms for hormonal control of adipose tissue lipolysis. Int J Obes. 1985;9:129–135. - PubMed
-
- Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005;54:3190–3197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

