Molecular dynamics of a thermostable multicopper oxidase from Thermus thermophilus HB27: structural differences between the apo and holo forms
- PMID: 22808237
- PMCID: PMC3393687
- DOI: 10.1371/journal.pone.0040700
Molecular dynamics of a thermostable multicopper oxidase from Thermus thermophilus HB27: structural differences between the apo and holo forms
Abstract
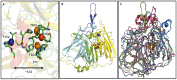
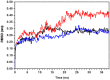
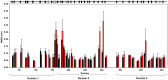
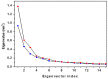
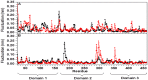
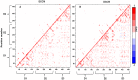
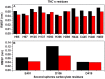
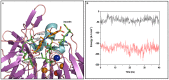
Molecular dynamic (MD) simulations have been performed on Tth-MCO, a hyperthermophilic multicopper oxidase from thermus thermophilus HB27, in the apo as well as the holo form, with the aim of exploring the structural dynamic properties common to the two conformational states. According to structural comparison between this enzyme and other MCOs, the substrate in process to electron transfer in an outer-sphere event seems to transiently occupy a shallow and overall hydrophobic cavity near the Cu type 1 (T1Cu). The linker connecting the β-strands 21 and 24 of the second domain (loop (β21-β24)(D2)) has the same conformation in both states, forming a flexible lid at the entrance of the electron-transfer cavity. Loop (β21-β24)(D2) has been tentatively assigned a role occluding the access to the electron-transfer site. The dynamic of the loop (β21-β24)(D2) has been investigated by MD simulation, and results show that the structures of both species have the same secondary and tertiary structure during almost all the MD simulations. In the simulation, loop (β21-β24)(D2) of the holo form undergoes a higher mobility than in the apo form. In fact, loop (β21-β24)(D2) of the holo form experiences a conformational change which enables exposure to the electron-transfer site (open conformation), while in the apo form the opposite effect takes place (closed conformation). To confirm the hypothesis that the open conformation might facilitate the transient electron-donor molecule occupation of the site, the simulation was extended another 40 ns with the electron-donor molecule docked into the protein cavity. Upon electron-donor molecule stabilization, loops near the cavity reduce their mobility. These findings show that coordination between the copper and the protein might play an important role in the general mobility of the enzyme, and that the open conformation seems to be required for the electron transfer process to T1Cu.
Conflict of interest statement
Figures

Similar articles
-
Thermostable multicopper oxidase from Thermus thermophilus HB27: crystallization and preliminary X-ray diffraction analysis of apo and holo forms.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Dec 1;67(Pt 12):1595-8. doi: 10.1107/S174430911103805X. Epub 2011 Nov 26. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 22139175 Free PMC article.
-
Simulation of the cavity-binding site of three bacterial multicopper oxidases upon complex stabilization: interactional profile and electron transference pathways.J Biomol Struct Dyn. 2014;32(8):1303-17. doi: 10.1080/07391102.2013.817954. Epub 2013 Jul 16. J Biomol Struct Dyn. 2014. PMID: 23859715
-
X-ray-induced catalytic active-site reduction of a multicopper oxidase: structural insights into the proton-relay mechanism and O2-reduction states.Acta Crystallogr D Biol Crystallogr. 2015 Dec 1;71(Pt 12):2396-411. doi: 10.1107/S1399004715018714. Epub 2015 Nov 26. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 26627648 Free PMC article.
-
Multicopper oxidases: a workshop on copper coordination chemistry, electron transfer, and metallophysiology.J Biol Inorg Chem. 2010 Jan;15(1):15-28. doi: 10.1007/s00775-009-0590-9. Epub 2009 Oct 9. J Biol Inorg Chem. 2010. PMID: 19816718 Review.
-
Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase.Chem Rec. 2007;7(4):220-9. doi: 10.1002/tcr.20125. Chem Rec. 2007. PMID: 17663447 Review.
Cited by
-
Activity-stability relationships revisited in blue oxidases catalyzing electron transfer at extreme temperatures.Extremophiles. 2016 Sep;20(5):621-9. doi: 10.1007/s00792-016-0851-9. Epub 2016 Jun 17. Extremophiles. 2016. PMID: 27315165
-
Overexpression of a novel thermostable and chloride-tolerant laccase from Thermus thermophilus SG0.5JP17-16 in Pichia pastoris and its application in synthetic dye decolorization.PLoS One. 2015 Mar 19;10(3):e0119833. doi: 10.1371/journal.pone.0119833. eCollection 2015. PLoS One. 2015. PMID: 25790466 Free PMC article.
-
Maturation of Rhodobacter capsulatus Multicopper Oxidase CutO Depends on the CopA Copper Efflux Pathway and Requires the cutF Product.Front Microbiol. 2021 Sep 8;12:720644. doi: 10.3389/fmicb.2021.720644. eCollection 2021. Front Microbiol. 2021. PMID: 34566924 Free PMC article.
-
Optical Monitoring of In Situ Iron Loading into Single, Native Ferritin Proteins.Nano Lett. 2023 Apr 26;23(8):3251-3258. doi: 10.1021/acs.nanolett.3c00042. Epub 2023 Apr 13. Nano Lett. 2023. PMID: 37053043 Free PMC article.
-
Structure-Function Relationship of the β-Hairpin of Thermus thermophilus HB27 Laccase.Int J Mol Sci. 2025 Jan 16;26(2):735. doi: 10.3390/ijms26020735. Int J Mol Sci. 2025. PMID: 39859450 Free PMC article.
References
-
- Solomon EI, Sundaram UM, Machonkin TE. Multicopper Oxidases and Oxygenases. Chem Rev. 1996;96:2563–2606. - PubMed
-
- Kosman DJ. Multicopper oxidases: a workshop on copper coordination chemistry, electron transfer, and metallophysiology. J Biol Inorg Chem. 2010;15:15–28. - PubMed
-
- Quintanar L, Stoj C, Taylor AB, Hart PJ, Kosman DJ, et al. Shall we dance? How a multicopper oxidase chooses its electron transfer partner. Acc Chem Res. 2007;40:445–452. - PubMed
-
- Messerschmidt A, Ladenstein R, Huber R, Bolognesi M, Avigliano L, et al. Refined crystal structure of ascorbate oxidase at 1.9 A resolution. J Mol Biol. 1992;224:179–205. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

