Developing cardiac and skeletal muscle share fast-skeletal myosin heavy chain and cardiac troponin-I expression
- PMID: 22808244
- PMCID: PMC3393685
- DOI: 10.1371/journal.pone.0040725
Developing cardiac and skeletal muscle share fast-skeletal myosin heavy chain and cardiac troponin-I expression
Abstract
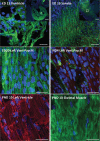
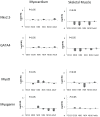
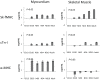
Skeletal muscle derived stem cells (MDSCs) transplanted into injured myocardium can differentiate into fast skeletal muscle specific myosin heavy chain (sk-fMHC) and cardiac specific troponin-I (cTn-I) positive cells sustaining recipient myocardial function. We have recently found that MDSCs differentiate into a cardiomyocyte phenotype within a three-dimensional gel bioreactor. It is generally accepted that terminally differentiated myocardium or skeletal muscle only express cTn-I or sk-fMHC, respectively. Studies have shown the presence of non-cardiac muscle proteins in the developing myocardium or cardiac proteins in pathological skeletal muscle. In the current study, we tested the hypothesis that normal developing myocardium and skeletal muscle transiently share both sk-fMHC and cTn-I proteins. Immunohistochemistry, western blot, and RT-PCR analyses were carried out in embryonic day 13 (ED13) and 20 (ED20), neonatal day 0 (ND0) and 4 (ND4), postnatal day 10 (PND10), and 8 week-old adult female Lewis rat ventricular myocardium and gastrocnemius muscle. Confocal laser microscopy revealed that sk-fMHC was expressed as a typical striated muscle pattern within ED13 ventricular myocardium, and the striated sk-fMHC expression was lost by ND4 and became negative in adult myocardium. cTn-I was not expressed as a typical striated muscle pattern throughout the myocardium until PND10. Western blot and RT-PCR analyses revealed that gene and protein expression patterns of cardiac and skeletal muscle transcription factors and sk-fMHC within ventricular myocardium and skeletal muscle were similar at ED20, and the expression patterns became cardiac or skeletal muscle specific during postnatal development. These findings provide new insight into cardiac muscle development and highlight previously unknown common developmental features of cardiac and skeletal muscle.
Conflict of interest statement
Figures



References
-
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. Journal of muscle research and cell motility. 1989;10:197–205. - PubMed
-
- Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Reviews of physiology, biochemistry and pharmacology. 1990;116:1–76. - PubMed
-
- Bortolotto SK, Cellini M, Stephenson DG, Stephenson GM. MHC isoform composition and Ca(2+)- or Sr(2+)-activation properties of rat skeletal muscle fibers. American journal of physiology Cell physiology. 2000;279:C1564–1577. - PubMed
-
- Apple FS. Tissue specificity of cardiac troponin I, cardiac troponin T and creatine kinase-MB. Clinica chimica acta; international journal of clinical chemistry. 1999;284:151–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

