Respiratory syncytial virus assembles into structured filamentous virion particles independently of host cytoskeleton and related proteins
- PMID: 22808269
- PMCID: PMC3396619
- DOI: 10.1371/journal.pone.0040826
Respiratory syncytial virus assembles into structured filamentous virion particles independently of host cytoskeleton and related proteins
Abstract
Respiratory syncytial virus (RSV) is a single-stranded RNA virus that assembles into viral filaments at the cell surface. Virus assembly often depends on the ability of a virus to use host proteins to accomplish viral tasks. Since the fusion protein cytoplasmic tail (FCT) is critical for viral filamentous assembly, we hypothesized that host proteins important for viral assembly may be recruited by the FCT. Using a yeast two-hybrid screen, we found that filamin A interacted with FCT, and mammalian cell experiments showed it localized to viral filaments but did not affect viral replication. Furthermore, we found that a number of actin-associated proteins also were excluded from viral filaments. Actin or tubulin cytoskeletal rearrangement was not necessary for F trafficking to the cell surface or for viral assembly into filaments, but was necessary for optimal viral replication and may be important for anchoring viral filaments. These findings suggest that RSV assembly into filaments occurs independently of actin polymerization and that viral proteins are the principal drivers for the mechanical tasks involved with formation of complex, structured RSV filaments at the host cell plasma membrane.
Conflict of interest statement
Figures
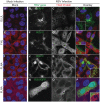
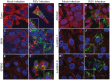
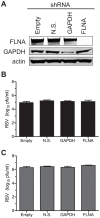

References
-
- Knipe DM, Howley PM editors. Fields Virology. Fifth. Knipe DM, Howley PM, editors Lippincott Williams & Wilkins. p. 2006.
-
- Harrison MS, Sakaguchi T, Schmitt AP. Paramyxovirus assembly and budding: building particles that transmit infections. Int J Biochem Cell Biol. 2010;42:1416–1429. doi: 10.1016/j.biocel.2010.04.005. - DOI - PMC - PubMed
-
- Brock SC, Goldenring JR, Crowe JE. Apical recycling systems regulate directional budding of respiratory syncytial virus from polarized epithelial cells. Proc Natl Acad Sci USA. 2003;100:15143–15148. doi: 10.1073/pnas.2434327100. - DOI - PMC - PubMed
-
- Lindquist ME, Lifland AW, Utley TJ, Santangelo PJ, Crowe JE. Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication. J Virol. 2010;84:12274–12284. doi: 10.1128/JVI.00260-10. - DOI - PMC - PubMed
-
- Li D, Jans DA, Bardin PG, Meanger J, Mills J, et al. Association of respiratory syncytial virus M protein with viral nucleocapsids is mediated by the M2-1 protein. J Virol. 2008;82:8863–8870. doi: 10.1128/JVI.00343-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK048370/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- NIH R01 DK48370/DK/NIDDK NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- P30CA068485/CA/NCI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- NIGMS/NIH T32 GM007347/PHS HHS/United States
- R01 DK070856/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

