Automated segmentation of retinal blood vessels in spectral domain optical coherence tomography scans
- PMID: 22808422
- PMCID: PMC3395475
- DOI: 10.1364/BOE.3.001478
Automated segmentation of retinal blood vessels in spectral domain optical coherence tomography scans
Abstract
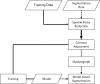
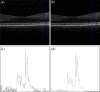
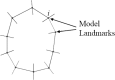
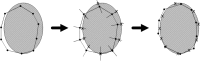
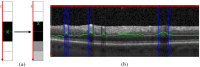
The correct segmentation of blood vessels in optical coherence tomography (OCT) images may be an important requirement for the analysis of intra-retinal layer thickness in human retinal diseases. We developed a shape model based procedure for the automatic segmentation of retinal blood vessels in spectral domain (SD)-OCT scans acquired with the Spectralis OCT system. The segmentation procedure is based on a statistical shape model that has been created through manual segmentation of vessels in a training phase. The actual segmentation procedure is performed after the approximate vessel position has been defined by a shadowgraph that assigns the lateral vessel positions. The active shape model method is subsequently used to segment blood vessel contours in axial direction. The automated segmentation results were validated against the manual segmentation of the same vessels by three expert readers. Manual and automated segmentations of 168 blood vessels from 34 B-scans were analyzed with respect to the deviations in the mean Euclidean distance and surface area. The mean Euclidean distance between the automatically and manually segmented contours (on average 4.0 pixels respectively 20 µm against all three experts) was within the range of the manually marked contours among the three readers (approximately 3.8 pixels respectively 18 µm for all experts). The area deviations between the automated and manual segmentation also lie within the range of the area deviations among the 3 clinical experts. Intra reader variability for the experts was between 0.9 and 0.94. We conclude that the automated segmentation approach is able to segment blood vessels with comparable accuracy as expert readers and will provide a useful tool in vessel analysis of whole C-scans, and in particular in multicenter trials.
Keywords: (100.0100) Image processing; (100.3008) Image recognition, algorithms and filters; (110.6880) Three-dimensional image acquisition; (170.4500) Optical coherence tomography.
Figures



References
-
- A. Budai, G. Michelson, and J. Hornegger, “Multiscale Blood Vessel Segmentation in Retinal Fundus Images,” in Proceedings of Bildverarbeitung für die Medizin (Springer Verlag, 2010), pp. 261–265.
-
- Niemeijer M., Garvin M. K., van Ginneken B., Sonka M., Abràmoff M. D., “Vessel segmentation in 3D spectral OCT scans of the retina,” Proc. SPIE 6914, 69141R–, 69141R-8. (2008). 10.1117/12.772680 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
