Image formation by linear and nonlinear digital scanned light-sheet fluorescence microscopy with Gaussian and Bessel beam profiles
- PMID: 22808423
- PMCID: PMC3395476
- DOI: 10.1364/BOE.3.001492
Image formation by linear and nonlinear digital scanned light-sheet fluorescence microscopy with Gaussian and Bessel beam profiles
Abstract
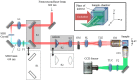
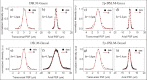
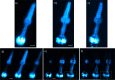
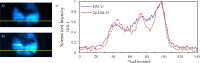
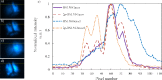
We present the implementation of a combined digital scanned light-sheet microscope (DSLM) able to work in the linear and nonlinear regimes under either Gaussian or Bessel beam excitation schemes. A complete characterization of the setup is performed and a comparison of the performance of each DSLM imaging modality is presented using in vivoCaenorhabditis elegans samples. We found that the use of Bessel beam nonlinear excitation results in better image contrast over a wider field of view.
Keywords: (140.3300) Laser beam shaping; (170.3880) Medical and biological imaging; (180.2520) Fluorescence microscopy; (180.4315) Nonlinear microscopy; (190.4180) Multiphoton processes.
Figures


References
-
- Alford W. J., VanderNeut R. D., Zaleckas V. J., “Laser scanning microscopy,” Proc. IEEE 70(6), 641–651 (1982). 10.1109/PROC.1982.12362 - DOI
-
- J. B. Pawley, ed., Handbook of Biological Confocal Microscopy, 3rd. ed. (Springer, 2006), pp. 680–689.
LinkOut - more resources
Full Text Sources
Other Literature Sources
