Attention deficit hyperactivity disorder subtypes and symptom response in adults treated with lisdexamfetamine dimesylate
- PMID: 22808446
- PMCID: PMC3398683
Attention deficit hyperactivity disorder subtypes and symptom response in adults treated with lisdexamfetamine dimesylate
Abstract
Objective: To evaluate the efficacy of lisdexamfetamine dimesylate in adults with attention deficit hyperactivity disorder symptom subtypes who exhibit predominantly inattention, hyperactivity/ impulsivity, or combined symptom clusters.
Design/setting/participants: This is a post-hoc analysis from a multicenter, one-year, open-label lisdexamfetamine dimesylate study in adults with attention deficit hyperactivity disorder previously completing two weeks or more in a four-week, randomized, placebo-controlled lisdexamfetamine dimesylate study, using Attention Deficit Hyperactivity Disorder Rating Scale IV symptom ratings as an attention deficit hyperactivity disorder subtype proxy (N=349).
Measurements: Attention Deficit Hyperactivity Disorder Rating Scale IV was measured at baseline of prior study and throughout the open-label study. Proxy subtypes were based on item scores of 2 (moderate) or 3 (severe), representing endorsement of at least six of nine symptoms on respective subscales; predominantly combined type endorsed at least six of nine symptoms on each subscale. Overall safety evaluations included treatment-emergent adverse events.
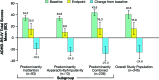
Results: At baseline, 93 of 345 participants exhibited predominantly inattention, 13 predominantly hyperactivity/ impulsivity, 236 combined symptom clusters, and three were unassigned. For the three subgroups, respectively, mean (standard deviation) Attention Deficit Hyperactivity Disorder Rating Scale IV total scores at baseline were 34.5 (4.02), 33.8 (3.27), and 43.6 (5.24); change from baseline to endpoint scores were -19.3 (9.48), -24.0 (7.22), and -27.3 (11.78). Mean (standard deviation) end-of-study lisdexamfetamine dimesylate dose was 57.7 (14.75), 53.1 (16.01), and 56.9 (14.94)mg/day, respectively.Treatment-emergent adverse events (>5%) were upper respiratory tract infection (21.8%), insomnia (19.5%), headache (17.2%), dry mouth (16.6%), decreased appetite (14.3%), irritability (11.2%), anxiety (8.3%), nasopharyngitis (7.4%), sinusitis (6.6%), decreased weight (6.0%), back pain (5.4%), and muscle spasms (5.2%).
Conclusions: Lisdexamfetamine dimesylate was effective in participants with predominantly inattention, hyperactivity/ impulsivity, and combined attention deficit hyperactivity disorder symptom clusters. Groups exhibiting specific predominant subtype symptoms did not differ in clinical response to lisdexamfetamine dimesylate.
Keywords: ADHD-RS-IV symptom subtype; Lisdexamfetamine dimesylate (LDX); amphetamine; attention-deficit/hyperactivity disorder (ADHD), adults; clinical response; predominantly combined; predominantly hyperactivity/impulsivity; predominantly inattention; stimulant.
Figures

References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Text Revision. Washington, DC: American Psychiatric Press Inc.; 2000. Attention-deficit and disruptive behavior disorders; pp. 85–93.
-
- Landgraf JM. Monitoring quality of life in adults with ADHD: reliability and validity of a new measure. J Atten Disord. 2007;11(3):351–362. - PubMed
-
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111(2):279–289. - PubMed
-
- Millstein RB, Wilens TE, Biederman J, Spencer TJ. Presenting ADHD symptoms and subtypes in clinically referred adults with ADHD. J Atten Disord. 1997;2(3):159–166.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
