Enzymes for the homeland defense: optimizing phosphotriesterase for the hydrolysis of organophosphate nerve agents
- PMID: 22809162
- PMCID: PMC3447986
- DOI: 10.1021/bi300811t
Enzymes for the homeland defense: optimizing phosphotriesterase for the hydrolysis of organophosphate nerve agents
Abstract
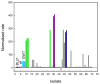
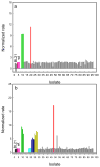
Phosphotriesterase (PTE) from soil bacteria is known for its ability to catalyze the detoxification of organophosphate pesticides and chemical warfare agents. Most of the organophosphate chemical warfare agents are a mixture of two stereoisomers at the phosphorus center, and the S(P)-enantiomers are significantly more toxic than the R(P)-enantiomers. In previous investigations, PTE variants were created through the manipulation of the substrate binding pockets and these mutants were shown to have greater catalytic activities for the detoxification of the more toxic S(P)-enantiomers of nerve agent analogues for GB, GD, GF, VX, and VR than the less toxic R(P)-enantiomers. In this investigation, alternate strategies were employed to discover additional PTE variants with significant improvements in catalytic activities relative to that of the wild-type enzyme. Screening and selection techniques were utilized to isolate PTE variants from randomized libraries and site specific modifications. The catalytic activities of these newly identified PTE variants toward the S(P)-enantiomers of chromophoric analogues of GB, GD, GF, VX, and VR have been improved up to 15000-fold relative to that of the wild-type enzyme. The X-ray crystal structures of the best PTE variants were determined. Characterization of these mutants with the authentic G-type nerve agents has confirmed the expected improvements in catalytic activity against the most toxic enantiomers of GB, GD, and GF. The values of k(cat)/K(m) for the H257Y/L303T (YT) mutant for the hydrolysis of GB, GD, and GF were determined to be 2 × 10(6), 5 × 10(5), and 8 × 10(5) M(-1) s(-1), respectively. The YT mutant is the most proficient enzyme reported thus far for the detoxification of G-type nerve agents. These results support a combinatorial strategy of rational design and directed evolution as a powerful tool for the discovery of more efficient enzymes for the detoxification of organophosphate nerve agents.
Figures





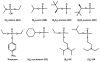



References
-
- Dumas DP, Durst HD, Landis WG, Raushel FM, Wild JR. Inactivation of organophosphorus nerve agents by the phosphotriesterase from Pseudomonas diminuta. Arch Biochem Biophys. 1990;277:155–159. - PubMed
-
- Lai K, Grimsley JK, Kuhlmann BD, Scapozza L, Harvey SP, DeFrank JJ, Kolakowski JE, Wild JR. Rational enzyme design: Computer modeling and site-directed mutagenesis for the modification of catalytic specificity in organophosphorus hydrolase. Chimia. 1996;50:430–431.
-
- Raveh L, Segall Y, Leader H, Rothschild N, Levanon D, Henis Y, Ashani Y. Protection against tabun toxicity in mice by prophylaxis with an enzyme hydrolyzing organophosphate esters. Biochem Pharma. 1992;22:397–400. - PubMed
-
- Caldwell SR, Newcomb JR, Schlecht KA, Raushel FM. Limits of diffusion in the hydrolysis of substrates by the phosphotriesterase from Pseudomonas diminuta. Biochemistry. 1991;30:7438–7444. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

