Emerging role of radiolabeled nanoparticles as an effective diagnostic technique
- PMID: 22809406
- PMCID: PMC3441881
- DOI: 10.1186/2191-219X-2-39
Emerging role of radiolabeled nanoparticles as an effective diagnostic technique
Abstract
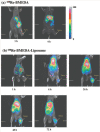
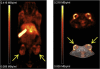
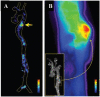
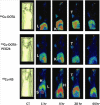
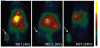
Nanomedicine is emerging as a promising approach for diagnostic applications. Nanoparticles are structures in the nanometer size range, which can present different shapes, compositions, charges, surface modifications, in vitro and in vivo stabilities, and in vivo performances. Nanoparticles can be made of materials of diverse chemical nature, the most common being metals, metal oxides, silicates, polymers, carbon, lipids, and biomolecules. Nanoparticles exist in various morphologies, such as spheres, cylinders, platelets, and tubes. Radiolabeled nanoparticles represent a new class of agent with great potential for clinical applications. This is partly due to their long blood circulation time and plasma stability. In addition, because of the high sensitivity of imaging with radiolabeled compounds, their use has promise of achieving accurate and early diagnosis. This review article focuses on the application of radiolabeled nanoparticles in detecting diseases such as cancer and cardiovascular diseases and also presents an overview about the formulation, stability, and biological properties of the nanoparticles used for diagnostic purposes.
Figures





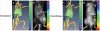

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

