Cell cycle-regulated cortical dynein/dynactin promotes symmetric cell division by differential pole motion in anaphase
- PMID: 22809624
- PMCID: PMC3431930
- DOI: 10.1091/mbc.E12-02-0109
Cell cycle-regulated cortical dynein/dynactin promotes symmetric cell division by differential pole motion in anaphase
Abstract
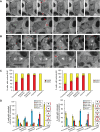
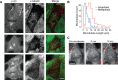
In cultured mammalian cells, how dynein/dynactin contributes to spindle positioning is poorly understood. To assess the role of cortical dynein/dynactin in this process, we generated mammalian cell lines expressing localization and affinity purification (LAP)-tagged dynein/dynactin subunits from bacterial artificial chromosomes and observed asymmetric cortical localization of dynein and dynactin during mitosis. In cells with asymmetrically positioned spindles, dynein and dynactin were both enriched at the cortex distal to the spindle. NuMA, an upstream targeting factor, localized asymmetrically along the cell cortex in a manner similar to dynein and dynactin. During spindle motion toward the distal cortex, dynein and dynactin were locally diminished and subsequently enriched at the new distal cortex. At anaphase onset, we observed a transient increase in cortical dynein, followed by a reduction in telophase. Spindle motion frequently resulted in cells entering anaphase with an asymmetrically positioned spindle. These cells gave rise to symmetric daughter cells by dynein-dependent differential spindle pole motion in anaphase. Our results demonstrate that cortical dynein and dynactin dynamically associate with the cell cortex in a cell cycle-regulated manner and are required to correct spindle mispositioning in LLC-Pk1 epithelial cells.
Figures





References
-
- Busson S, Dujardin D, Moreau A, Dompierre J, De Mey JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol. 1998;8:541–544. - PubMed
-
- Cheeseman IM, Desai A. A combined approach for the localization and tandem affinity purification of protein complexes from metazoans. Sci STKE. 2005;2005:pl1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

