Lesion-specific DNA-binding and repair activities of human O⁶-alkylguanine DNA alkyltransferase
- PMID: 22810209
- PMCID: PMC3467069
- DOI: 10.1093/nar/gks674
Lesion-specific DNA-binding and repair activities of human O⁶-alkylguanine DNA alkyltransferase
Abstract
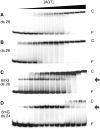
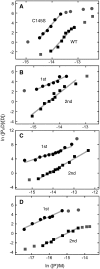
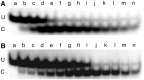
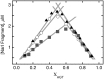
Binding experiments with alkyl-transfer-active and -inactive mutants of human O(6)-alkylguanine DNA alkyltransferase (AGT) show that it forms an O(6)-methylguanine (6mG)-specific complex on duplex DNA that is distinct from non-specific assemblies previously studied. Specific complexes with duplex DNA have a 2:1 stoichiometry that is formed without accumulation of a 1:1 intermediate. This establishes a role for cooperative interactions in lesion binding. Similar specific complexes could not be detected with single-stranded DNA. The small difference between specific and non-specific binding affinities strongly limits the roles that specific binding can play in the lesion search process. Alkyl-transfer kinetics with a single-stranded substrate indicate that two or more AGT monomers participate in the rate-limiting step, showing for the first time a functional link between cooperative binding and the repair reaction. Alkyl-transfer kinetics with a duplex substrate suggest that two pathways contribute to the formation of the specific 6mG-complex; one at least first order in AGT, we interpret as direct lesion binding. The second, independent of [AGT], is likely to include AGT transfer from distal sites to the lesion in a relatively slow unimolecular step. We propose that transfer between distal and lesion sites is a critical step in the repair process.
Figures








Similar articles
-
Repair of O6-methylguanine adducts in human telomeric G-quadruplex DNA by O6-alkylguanine-DNA alkyltransferase.Nucleic Acids Res. 2014 Sep;42(15):9781-91. doi: 10.1093/nar/gku659. Epub 2014 Jul 30. Nucleic Acids Res. 2014. PMID: 25080506 Free PMC article.
-
Quaternary interactions and supercoiling modulate the cooperative DNA binding of AGT.Nucleic Acids Res. 2017 Jul 7;45(12):7226-7236. doi: 10.1093/nar/gkx223. Nucleic Acids Res. 2017. PMID: 28575445 Free PMC article.
-
Investigation of the role of tyrosine-114 in the activity of human O6-alkylguanine-DNA alkyltranferase.Biochemistry. 1998 Sep 8;37(36):12489-95. doi: 10.1021/bi9811718. Biochemistry. 1998. PMID: 9730821
-
Insight into the cooperative DNA binding of the O⁶-alkylguanine DNA alkyltransferase.DNA Repair (Amst). 2014 Aug;20:14-22. doi: 10.1016/j.dnarep.2014.01.006. Epub 2014 Feb 16. DNA Repair (Amst). 2014. PMID: 24553127 Free PMC article. Review.
-
Conserved structural motifs governing the stoichiometric repair of alkylated DNA by O(6)-alkylguanine-DNA alkyltransferase.Mutat Res. 2000 Aug 30;460(3-4):151-63. doi: 10.1016/s0921-8777(00)00024-0. Mutat Res. 2000. PMID: 10946226 Review.
Cited by
-
Structure-function relationships governing activity and stability of a DNA alkylation damage repair thermostable protein.Nucleic Acids Res. 2015 Oct 15;43(18):8801-16. doi: 10.1093/nar/gkv774. Epub 2015 Jul 30. Nucleic Acids Res. 2015. PMID: 26227971 Free PMC article.
-
Alkyltransferase-like protein clusters scan DNA rapidly over long distances and recruit NER to alkyl-DNA lesions.Proc Natl Acad Sci U S A. 2020 Apr 28;117(17):9318-9328. doi: 10.1073/pnas.1916860117. Epub 2020 Apr 9. Proc Natl Acad Sci U S A. 2020. PMID: 32273391 Free PMC article.
-
The DNA Alkyltransferase Family of DNA Repair Proteins: Common Mechanisms, Diverse Functions.Int J Mol Sci. 2023 Dec 29;25(1):463. doi: 10.3390/ijms25010463. Int J Mol Sci. 2023. PMID: 38203633 Free PMC article. Review.
-
Repair of O6-methylguanine adducts in human telomeric G-quadruplex DNA by O6-alkylguanine-DNA alkyltransferase.Nucleic Acids Res. 2014 Sep;42(15):9781-91. doi: 10.1093/nar/gku659. Epub 2014 Jul 30. Nucleic Acids Res. 2014. PMID: 25080506 Free PMC article.
-
Resolving the subtle details of human DNA alkyltransferase lesion search and repair mechanism by single-molecule studies.Proc Natl Acad Sci U S A. 2022 Mar 15;119(11):e2116218119. doi: 10.1073/pnas.2116218119. Epub 2022 Mar 8. Proc Natl Acad Sci U S A. 2022. PMID: 35259021 Free PMC article.
References
-
- Kyrtopoulos SA, Anderson LM, Chhabra SK, Souliotis VL, Pletsa V, Valavanis C, Georgiadis P. DNA adducts and the mechanism of carcinogenesis and cytotoxicity of methylating agents of environmental and clinical significance. Cancer Detect. Prev. 1997;21:391–405. - PubMed
-
- Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J. Clin. Oncol. 2002;20:2388–2399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

