Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons
- PMID: 22810227
- PMCID: PMC3436277
- DOI: 10.1074/jbc.M112.374405
Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons
Abstract
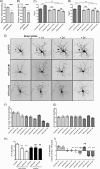
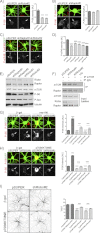
Dendrites are the main site of information input into neurons. Their development is a multistep process controlled by mammalian target of rapamycin (mTOR) among other proteins. mTOR is a serine/threonine protein kinase that forms two functionally distinct complexes in mammalian cells: mTORC1 and mTORC2. However, the one that contributes to mammalian neuron development remains unknown. This work used short hairpin RNA against Raptor and Rictor, unique components of mTORC1 and mTORC2, respectively, to dissect mTORC involvement in this process. We provide evidence that both mTOR complexes are crucial for the proper dendritic arbor morphology of hippocampal neurons. These two complexes are required for dendritic development both under basal conditions and upon the induction of mTOR-dependent dendritic growth. We also identified Akt as a downstream effector of mTORC2 needed for proper dendritic arbor morphology, the action of which required mTORC1 and p70S6K1.
Figures






References
-
- Stuart G., Spruston N., Häusser M. (2007) Dendrites, 2nd Ed., Oxford University Press, Oxford
-
- Urbanska M., Blazejczyk M., Jaworski J. (2008) Molecular basis of dendritic arborization. Acta Neurobiol. Exp. (Wars) 68, 264–288 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

