The use of individualized tumor response testing in treatment selection: second randomization results from the LRF CLL4 trial and the predictive value of the test at trial entry
- PMID: 22810506
- PMCID: PMC3567236
- DOI: 10.1038/leu.2012.209
The use of individualized tumor response testing in treatment selection: second randomization results from the LRF CLL4 trial and the predictive value of the test at trial entry
Figures
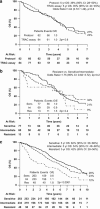
References
-
- Bosanquet AG, Johnson SA, Richards SM. Prognosis for fludarabine therapy of chronic lymphocytic leukaemia based on ex vivo drug response by DiSC assay. Br J Haematol. 1999;106:71–77. - PubMed
-
- Bosanquet AG, Richards SM, Wade R, Else M, Matutes E, Dyer MJS, et al. Drug cross-resistance and therapy-induced resistance in chronic lymphocytic leukaemia by an enhanced method of individualised tumour response testing. Br J Haematol. 2009;146:384–395. - PubMed
-
- Catovsky D, Richards S, Matutes E, Oscier D, Dyer MJS, Bezares RF, on behalf of the UK National Cancer Research Institute (NCRI) Haematological Oncology Clinical Studies Group and NCRI Chronic Lymphocytic Leukaemia Working Group et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–239. - PubMed
-
- Oscier D, Wade R, Davis Z, Morilla A, Best G, Richards S, et al. for the UK National Cancer Research Institute (NCRI) Chronic Lymphocytic Leukaemia Working Group. Biological risk factors identify 3 risk groups in the LRF CLL4 trial independent of treatment allocation. Hematologica. 2010;95:1705–1712. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

