Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2
- PMID: 22811423
- PMCID: PMC3893888
- DOI: 10.1152/physrev.00030.2011
Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2
Abstract
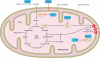
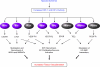
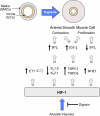
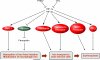
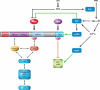
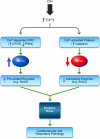
Hypoxia is a fundamental stimulus that impacts cells, tissues, organs, and physiological systems. The discovery of hypoxia-inducible factor-1 (HIF-1) and subsequent identification of other members of the HIF family of transcriptional activators has provided insight into the molecular underpinnings of oxygen homeostasis. This review focuses on the mechanisms of HIF activation and their roles in physiological and pathophysiological responses to hypoxia, with an emphasis on the cardiorespiratory systems. HIFs are heterodimers comprised of an O(2)-regulated HIF-1α or HIF-2α subunit and a constitutively expressed HIF-1β subunit. Induction of HIF activity under conditions of reduced O(2) availability requires stabilization of HIF-1α and HIF-2α due to reduced prolyl hydroxylation, dimerization with HIF-1β, and interaction with coactivators due to decreased asparaginyl hydroxylation. Stimuli other than hypoxia, such as nitric oxide and reactive oxygen species, can also activate HIFs. HIF-1 and HIF-2 are essential for acute O(2) sensing by the carotid body, and their coordinated transcriptional activation is critical for physiological adaptations to chronic hypoxia including erythropoiesis, vascularization, metabolic reprogramming, and ventilatory acclimatization. In contrast, intermittent hypoxia, which occurs in association with sleep-disordered breathing, results in an imbalance between HIF-1α and HIF-2α that causes oxidative stress, leading to cardiorespiratory pathology.
Figures




References
-
- Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res. 2006;66:5641–5647. - PubMed
-
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv21, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL076537/HL/NHLBI NIH HHS/United States
- HL-86493/HL/NHLBI NIH HHS/United States
- P01-HL-65608/HL/NHLBI NIH HHS/United States
- U54 CA143868/CA/NCI NIH HHS/United States
- HL-76537/HL/NHLBI NIH HHS/United States
- R01-HL-55338/HL/NHLBI NIH HHS/United States
- U54-CA-143868/CA/NCI NIH HHS/United States
- P01 HL025830/HL/NHLBI NIH HHS/United States
- P01 HL090554/HL/NHLBI NIH HHS/United States
- P20 GM078494/GM/NIGMS NIH HHS/United States
- P01 HL065608/HL/NHLBI NIH HHS/United States
- P20-GM-78494/GM/NIGMS NIH HHS/United States
- HL-90554/HL/NHLBI NIH HHS/United States
- R01 HL086493/HL/NHLBI NIH HHS/United States
- HHS-N268201000032C/PHS HHS/United States
- R01 HL055338/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

