Sirtuin 1 and sirtuin 3: physiological modulators of metabolism
- PMID: 22811431
- PMCID: PMC3746174
- DOI: 10.1152/physrev.00022.2011
Sirtuin 1 and sirtuin 3: physiological modulators of metabolism
Abstract
The sirtuins are a family of highly conserved NAD(+)-dependent deacetylases that act as cellular sensors to detect energy availability and modulate metabolic processes. Two sirtuins that are central to the control of metabolic processes are mammalian sirtuin 1 (SIRT1) and sirtuin 3 (SIRT3), which are localized to the nucleus and mitochondria, respectively. Both are activated by high NAD(+) levels, a condition caused by low cellular energy status. By deacetylating a variety of proteins that induce catabolic processes while inhibiting anabolic processes, SIRT1 and SIRT3 coordinately increase cellular energy stores and ultimately maintain cellular energy homeostasis. Defects in the pathways controlled by SIRT1 and SIRT3 are known to result in various metabolic disorders. Consequently, activation of sirtuins by genetic or pharmacological means can elicit multiple metabolic benefits that protect mice from diet-induced obesity, type 2 diabetes, and nonalcoholic fatty liver disease.
Figures
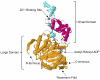

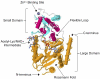
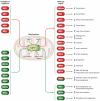
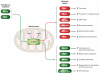


References
-
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. - PubMed
-
- Afshar G, Murnane JP. Characterization of a human gene with sequence homology to Saccharomyces cerevisiae SIR2. Gene. 1999;234:161–168. - PubMed
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

