An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents
- PMID: 22811462
- PMCID: PMC3429704
- DOI: 10.1161/STROKEAHA.112.658997
An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents
Abstract
Background and purpose: Tissue plasminogen activator (tPA) is the only approved therapy for acute ischemic stroke. However, tPA has a brief therapeutic window. Its side effects include intracerebral bleeding and neurotoxicity. Therefore, a combination therapy with tPA and agents that can extend the therapeutic window of tPA and/or counteract its side effects are warranted. Here, we studied whether 3K3A-APC, a neuroprotective analog of activated protein C with reduced anticoagulant activity, can enhance the therapeutic effects of tPA in models of ischemic stroke in rodents.
Methods: Human recombinant tPA (10 mg/kg), alone or in combination with human recombinant 3K3A-APC (2 mg/kg), was administered intravenously 4 hours after proximal or distal transient middle cerebral artery occlusion in mice and embolic stroke in rats. The 3K3A-APC was additionally administered for 3 to 4 consecutive days after stroke. The neuropathological and neurological analyses were performed at 1 to 7 days after stroke.
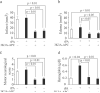
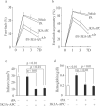
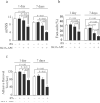
Results: In all models, tPA alone had no effects on the infarct volume or behavior (ie, neurological score, foot-fault, forelimb asymmetry, adhesive removal) compared with vehicle. The tPA and 3K3A-APC combination therapy reduced the infarct volume 24 hours and 7 days after proximal or distal transient middle cerebral artery occlusion in mice and 7 days after embolic stroke in rats by 65%, 63%, and 52%, respectively, significantly (P<0.05) improved behavior and eliminated tPA-induced intracerebral microhemorrhages.
Conclusions: The 3K3A-APC extends the therapeutic window of tPA for ischemic stroke in rodents. Therefore, this combination therapy also should be considered for treating stroke in humans.
Figures

References
-
- Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National us estimates of recombinant tissue plasminogen activator use: Icd-9 codes substantially underestimate. Stroke. 2008;39:924–928. - PubMed
-
- Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, et al. Activated protein c inhibits tissue plasminogen activator-induced brain hemorrhage. Nature medicine. 2006;12:1278–1285. - PubMed
-
- Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. The ninds t-pa stroke study group. Stroke. 1997;28:2109–2118. - PubMed
-
- Saver JL, Levine SR. Alteplase for ischaemic stroke--much sooner is much better. Lancet. 2010;375:1667–1668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

